Introduction
Consumers discriminate against meat that appears brown in color because they perceive this to indicate that the product is spoiled or no longer fresh (Font-i-Furnols and Guerrero, 2014). This discoloration results in reduced willingness to purchase and greater food waste. Within the United States alone, beef discoloration results in an estimated $3 billion lost value annually (Ramanathan et al., 2021), comprising nearly 2% of the $123 billion total revenue from US beef products in 2020 (USDA, 2021). Although a similar economic analysis is not available for pork, fresh pork does discolor (Zhu and Brewer, 1998a; King et al., 2011b). Although discoloration-related economic losses for fresh pork have not been quantified, if this same 2% reduction in total revenue were applied to the $24.9 billion estimated for US pork products (Cook and Schulz, 2022), pork discoloration could account for nearly $500 million in lost revenue. Therefore, antemortem and postmortem interventions to improve fresh pork color stability would increase consumers’ likelihood of purchase and maximize value.
Previous research has reported that pork loins could be selected based on expected color stability at 1 d postmortem with color-stable loins being darker and less yellow and having smaller hue angles than color-labile loins (King et al., 2011b). However, published data evaluating discoloration of other pork muscles is limited. In beef, it is well-established that muscles differ in color stability (McKenna et al., 2005; King et al., 2011a; Suman et al., 2014). However, limited research has evaluated traits predictive of greater color stability, especially in muscles other than the longissimus. Based on differences in color stability between muscles, it is likely that traits predictive of loin color stability may not be predictive of color stability in other pork muscles, especially muscles that are biochemically different.
Although other pork muscles are not commonly sold in the US using overwrap packaging, as is typical of loin chops (Mills, 2021), muscle color stability likely has implications for other product types. For example, the use of color-labile muscles in ground beef can have detrimental effects on color stability (Raines et al., 2010). With nearly 30% of pork in the US sold as sausage or fresh cuts other than loin chops or ribs (NPB, 2011), there is value in determining factors that can improve color stability of muscles other than the longissimus dorsi (LD). Identifying traits on day 1 of retail display that are indicative of improved color stability for multiple pork muscles could allow for genetic selection of these traits to produce pork with improved color stability, resulting in increased profits for the pork industry. Because the color stability of LD, triceps brachii (TB), and psoas major (PM) is well documented in beef, these muscles were chosen for the present study to characterize relationships in pork.
Therefore, the objectives of this study were to determine relationships between initial retail display traits and final discoloration in LD, TB, and PM and to determine what combination of initial traits was most predictive of final discoloration in each muscle. It was hypothesized that initial traits would be related to final measures of discoloration and that the combination of traits that were most predictive of improved color stability would differ between muscles.
Materials and Methods
Pigs used in this study were the offspring of sire lines representing Piétrain ancestry (Choice Genetics USA, West Des Moines, IA). Boars were mated to Camborough sows (PIC, Hendersonville, TN), and parity of the females was balanced among sire lines. Pigs were housed in single-sex pens by sire line with 4 pigs housed per pen. A total of 80 pens were allocated for another experiment, and the second heaviest pig in 20 pens was selected for slaughter. All pigs received an industry-typical corn-soy finishing diet. All protocols were approved by the University of Illinois Institutional Animal Care and Use Committee (Protocol #20095).
Sample preparation
Pigs (n = 20) were transported to the University of Illinois Meat Science Laboratory and held in lairage for approximately 16 h with no access to feed and ad libitum access to water. Pigs used in this study were slaughtered under the supervision of the US Department of Agriculture Food and Safety Inspection Service. Animals were immobilized via electrical stunning and terminated via exsanguination, eviscerated, and then allowed to chill at 4°C for approximately 22 h. After chilling, left sides of carcasses were fabricated to yield a picnic shoulder (NAMP #405) and bone-in loin (NAMP #410). Picnic shoulders were further fabricated to yield TB long head, and bone-in loins were fabricated to yield LD and PM. TB and PM were vacuum-packaged individually and aged for 21 d at 4°C. LD was cut into 2.54-cm chops using a push-feed slicer (Treif model 700 F, Oberlahr, Germany). The 3 chops immediately posterior to the area of the 10th rib were vacuum-packaged together and stored in the same manner as TB and PM until 21 d postmortem. After aging, TB and PM were manually cut with a knife to yield 3 chops measuring approximately 2.54 and 5.08 cm thick, respectively. Ultimate pH of the most anterior chop for each muscle was measured with a Hanna pH meter fitted with a glass-tipped electrode (model HI98163, Hanna Instruments, Smithfield, RI) calibrated using pH 4 and pH 7 buffer at 4°C.
Retail display
The 3 chops representing a single muscle and carcass were placed with the cut side up on a 27.3 × 14.9 cm polystyrene tray (Dyne-A-Pak, Laval, Quebec, Canada) with a soaker pad and overwrapped with polyvinyl chloride film (O2 transmission = 23,250 mL·m2·d−1, 72 gauge; Resinite packaging films, Borden, North Andover, MA). Each of the 3 chops was randomly allocated to day 1, day 3, or day 5 of display. Chops were removed from display after analysis on their respective days; day 1 chops were used for biochemical analysis, whereas day 3 and day 5 chops were used for a separate study. Packages were displayed in a standing display cooler with 4 shelves and rotated daily to minimize location effects on discoloration. Lighting was provided by 122 cm fluorescent bulbs (32 W, Octron XP, 6,500 K, Osram Sylvania, Wilmington, MA) positioned at the front of the display case. Samples were allowed to oxygenate for at least 2 h prior to color evaluations on day 1 of display. On day 1, samples were evaluated for subjective visual discoloration and instrumental surface color. Subjective visual discoloration of an entire package was evaluated through the film by a panel of 8 trained technicians using an unstructured line 10-cm line scale anchored at 0%, 50%, and 100% discoloration. Then, packaging was removed from chops, and instrumental surface color was evaluated using a HunterLab MiniScan EZ spectrophotometer (HunterLab, Reston, VA) and included CIE lightness (L*), redness (a*), and yellowness (b*) (CIE, 1976) and spectral data (400 to 700 nm) as described by King et al. (2023). The spectrophotometer used a D65 illuminant and open 31.8 mm aperture and was calibrated with a white and black tile specific to the machine. Instrumental L*, a*, and b* data were used to calculate chroma (measure of saturation: ) and hue angle overall measure of color: . Reflectance data were used to calculate the ratio of 630/580 nm (measure of brownness) and myoglobin (Mb) forms using the attenuance method as described by Krzywicki (1979). Reflectances at 474, 525, 572, and 700 nm were converted to attenuance values (A) using the equation A = log(1/R), where R = reflectance. Mb forms were calculated using the following equations:
Packages were rewrapped and returned to display after color measurements. On day 1 of display, subjective and instrumental discoloration were evaluated on all chops in a package. Discoloration evaluations for chops within a single package were averaged for statistical analyses. On day 5 of display, only 1 chop remained in each package and was evaluated for surface discoloration and 630/580 ratio in the same manner as on day 1.
Biochemical analyses
On day 1 of display, designated chops from each package were removed from display and trimmed of all external fat. Chops from LD and TB were cut in half perpendicular to the cut surface, and chops from PM were cut in half parallel to the cut surface. This was done to ensure muscle fibers were oriented in the same direction on the surface of each chop. One half of each sample was frozen at −80°C to be used for Mb quantification, whereas the remaining half was used for determination of oxygen consumption (OC) and metmyoglobin (MMb)-reducing activity (MRA). Sample portions reserved for OC and MRA were cut in half parallel to the muscle fibers, with the 2 halves randomly allotted to 1 analysis.
Oxygen consumption
OC was determined using the method outlined by King et al. (2023) with modifications. The freshly cut surface of the sample designated for OC was covered with clear oxygen-permeable film and allowed to oxygenate for 2 h at 4°C. After oxygenation, samples were vacuum-packaged and scanned immediately with the same Hunter spectrophotometer that was used for retail display evaluations. The Hunter was calibrated through the same material used for vacuum packaging. Samples were then allowed to incubate at 20°C for 1 h and then scanned a second time with the Hunter. The proportion of OMb from the initial and final scan was determined using the Krzywicki (1979) method as previously outlined. Percent OC was calculated using initial and final OMb content as outlined by King et al. (2023):
Metmyoglobin-reducing activity
MRA was determined using the method outlined by King et al. (2023) with modifications. The freshly cut surface of the sample designated for MRA was submerged in a solution of 0.3% sodium nitrite for 30 min at approximately 20°C. Samples were then removed from solution, blotted dry, and individually vacuum-packaged. Immediately after packaging, samples were scanned through the vacuum package with the same Hunter spectrophotometer that was used for OC. After scanning, samples were allowed to incubate for 3 h at 20°C. Samples were scanned a second time through the packaging after incubation was complete. The proportion of MMb from the initial and final scan was determined using the Krzywicki (1979) method as previously outlined. Percent MRA was calculated using initial and final MMb content as outlined by King et al. (2023):
Myoglobin content
Mb content was determined according to the procedure outlined by Faustman and Phillips (2001) with modifications. Samples were diced and pulverized into a fine powder using liquid nitrogen. Duplicate 5 g samples were homogenized with 45 mL ice cold mM potassium phosphate buffer (pH = 6.8) and filtered through Whatman no. 1 filter paper. After filtering, 150 μL of the filtrate was pipetted into a 96-well plate with potassium phosphate buffer as a blank. The absorbance of the filtrate was measured at 525 nm using a plate reader (Synergy HT Multimode Microplate Reader, BioTek, Winooski, VT). Mb content was determined using the following equation:
where A525 = absorbance at 525 nm, 7.6 mM−1·cm−1 =millimolar extinction coefficient of Mb at 525 nm, 1 cm = path length of cuvette, and 17,000 Da = average molecular mass of Mb. The dilution factor was 10.Statistical analyses
Summary statistics for initial (day 1) color and biochemical measurements were determined using the MEANS procedure of SAS (v. 9.4; SAS Institute, Cary, NC). Initial measurement differences were evaluated using the MIXED procedure with muscle type serving as a fixed effect and individual animal serving as a random effect. Differences in color and biochemical traits between muscles were considered different at P ≤ 0.05.
For each muscle, Pearson correlation coefficients (r) were determined between initial traits (color and biochemical) and final visual discoloration or 630/580 nm ratio using the CORR procedure. Comparisons of independent correlation coefficients between muscles were then achieved following the example of Kenny (1987) and Lowell et al. (2017) using z tests for comparing 2 independent correlations. Two muscles were compared at a time: LD versus TB, LD versus PM, and TB versus PM. Data were grouped into 3 data sets by muscle (LD, TB, and PM). For each data set, correlation coefficients were transformed using the Fisher’s r to z transformation with the FISHER option of the CORR procedure in SAS. The Fisher’s r to z transformation was performed using the following equation:
where r is the Pearson correlation coefficient and z is the transformed value of the correlation coefficient. This transformation was used to ensure that transformed coefficients were nearly normally distributed and that correlation variances were approximately the same regardless of the value of the population correlation (Kenny, 1987). If the z value was statistically significant, then correlations between 2 populations were different (Kenny, 1987). Fischer’s transformed z values were then merged into a single data set for each comparison (LD vs. TB, LD vs. PM, and TB vs. PM) using the following equation:Correlations for an individual muscle were considered weak (in absolute value) at r < 0.35, moderate at 0.36 ≤ r ≤ 0.67, and strong at r > 0.68. P values for comparisons between correlations for 2 muscles were reported when both muscles had a significant correlation (P > 0.05). Figures displaying correlations between an initial trait and final trait were created when 2 of the 3 muscles had significant correlations between those traits.
Linear stepwise regression equations were developed using independent candidate variables to predict final visual discoloration and final 630/580 nm ratio for each muscle. Multicollinearity among independent variables was assessed using a variance inflation factor (VIF) statistic. Parameters that exceeded a VIF of 10 were excluded from respective models. Variables included in the model for LD were L*, pH, a*, b*, OMb, OC, MRA, and Mb. Variables included in the model for TB were pH, a*, b*, OMb, OC, MRA, and Mb. Variables included in the model for PM were a*, b*, OC, MRA, and Mb. Influence of individual observations on estimation of regression parameters was evaluated using the difference in fits (DFFITS) statistic. Observations were considered to have excessive influence when DFFITS ≥ 2[(p/n)1/2], where p = the number of parameters and n = the total number of observations. Two observations met these criteria for the models predicting LD traits and were removed from the data set for those models. No other observations were removed. Using a stepwise selection method, independent variables were required to have an SLENTRY level = 0.50 to enter the model and SLSTAY level = 0.45 to remain in the model.
Results
Population summary statistics for color and biochemical measurements on day 1 of display are reported in Table 1. LD was the least variable muscle for several traits, as indicated by lesser coefficient of variation (CV) values, including ultimate pH, L*, b*, chroma, hue angle, OMb, MRA, and OC. Visual discoloration, deoxymyoglobin (DMb), and Mb content had larger CV values for all 3 muscles (>25); however, these were likely influenced by small overall means, meaning that this increased variability is likely not practically important (Livers, 1942). LD had a greater CV for OC (CV = 48.69) compared with TB (CV = 5.05) and PM (CV = 9.93), likely because LD had a larger range in values (range = 61.26) than TB (range =9.32) or PM (range = 9.47).
Population summary statistics for day 1 retail display color and biochemical traits for longissimus dorsi, triceps brachii , and psoas major
| Variable | Samples, n | Mean | Minimum | Maximum | Range | SD1 | CV2 |
|---|---|---|---|---|---|---|---|
| Longissimus dorsi | |||||||
| pH | 20 | 5.61 | 5.51 | 5.80 | 0.19 | 0.07 | 1.29 |
| Lightness, L* | 20 | 59.10 | 53.79 | 64.04 | 4.94 | 2.95 | 5.00 |
| Redness, a* | 20 | 11.52 | 9.33 | 13.22 | 1.70 | 0.93 | 8.11 |
| Yellowness, b* | 20 | 18.57 | 17.09 | 19.65 | 1.08 | 0.71 | 3.80 |
| Chroma3 | 20 | 21.87 | 19.48 | 23.11 | 1.24 | 0.92 | 4.23 |
| Hue angle4 | 20 | 58.24 | 54.60 | 61.46 | 3.22 | 1.89 | 3.25 |
| 630/580 nm | 20 | 2.83 | 2.47 | 3.24 | 0.41 | 0.23 | 8.19 |
| Visual discoloration, % | 18 | 1.81 | 0.00 | 9.78 | 7.97 | 2.65 | 146.34 |
| Metmyoglobin (MMb), %5 | 20 | 11.86 | 7.13 | 15.11 | 3.25 | 1.96 | 16.51 |
| Deoxymyoglobin (DMb), %6 | 20 | 3.83 | −4.50 | 15.94 | 12.11 | 5.10 | 133.02 |
| Oxymyoglobin (OMb), %7 | 20 | 84.30 | 73.60 | 91.12 | 6.82 | 4.23 | 5.02 |
| Oxygen consumption, % | 20 | 59.38 | 17.09 | 120.64 | 61.26 | 28.91 | 48.69 |
| MMb-reducing activity, % | 20 | 48.99 | 27.68 | 59.41 | 10.42 | 7.79 | 15.89 |
| Milligrams myoglobin/grams meat | 20 | 0.74 | 0.38 | 1.26 | 0.52 | 0.20 | 27.29 |
| Triceps brachii | |||||||
| pH | 20 | 5.90 | 5.59 | 6.57 | 0.67 | 0.22 | 3.76 |
| Lightness, L* | 20 | 44.51 | 40.15 | 48.83 | 4.32 | 2.65 | 5.96 |
| Redness, a* | 20 | 17.11 | 15.08 | 19.35 | 2.24 | 1.23 | 7.18 |
| Yellowness, b* | 20 | 17.17 | 14.25 | 19.86 | 2.69 | 1.48 | 8.61 |
| Chroma3 | 20 | 24.26 | 20.75 | 27.12 | 2.86 | 1.75 | 7.21 |
| Hue angle4 | 20 | 45.07 | 41.66 | 48.75 | 3.68 | 1.90 | 4.23 |
| 630/580 nm | 20 | 4.31 | 3.77 | 4.66 | 0.35 | 0.23 | 5.45 |
| Visual discoloration, % | 20 | 2.07 | 0.00 | 6.00 | 3.93 | 1.89 | 91.27 |
| MMb, %5 | 20 | 19.29 | 17.50 | 20.93 | 1.65 | 0.92 | 4.79 |
| DMb, %6 | 20 | 13.64 | 0.81 | 36.26 | 22.62 | 8.99 | 65.90 |
| OMb, %7 | 20 | 67.07 | 43.88 | 80.22 | 13.15 | 9.02 | 13.45 |
| Oxygen consumption, % | 20 | 99.57 | 86.95 | 108.89 | 9.32 | 5.02 | 5.05 |
| MMb-reducing activity, % | 20 | 40.61 | 15.30 | 51.74 | 11.13 | 8.64 | 21.27 |
| Milligrams myoglobin/grams meat | 20 | 2.07 | 1.02 | 3.83 | 1.76 | 0.79 | 37.87 |
| Psoas major | |||||||
| pH | 20 | 5.84 | 5.58 | 6.40 | 0.56 | 0.24 | 4.08 |
| Lightness, L* | 20 | 47.41 | 42.01 | 55.34 | 7.93 | 3.67 | 7.75 |
| Redness, a* | 20 | 18.43 | 15.96 | 20.16 | 1.73 | 1.16 | 6.31 |
| Yellowness, b* | 20 | 19.43 | 15.12 | 21.18 | 1.75 | 1.76 | 9.07 |
| Chroma3 | 20 | 26.80 | 21.99 | 29.07 | 2.27 | 1.88 | 7.03 |
| Hue angle4 | 20 | 46.44 | 43.24 | 50.37 | 3.93 | 2.06 | 4.43 |
| 630/580 nm | 20 | 4.36 | 3.66 | 4.90 | 0.54 | 0.31 | 7.12 |
| Visual discoloration, % | 20 | 3.29 | 0.22 | 9.11 | 5.82 | 2.97 | 90.38 |
| MMb, %5 | 20 | 17.00 | 15.29 | 19.28 | 2.28 | 1.07 | 6.27 |
| DMb, %6 | 20 | 6.33 | −3.61 | 36.49 | 30.16 | 10.43 | 164.85 |
| OMb, %7 | 20 | 76.67 | 45.55 | 87.58 | 10.91 | 10.58 | 13.79 |
| Oxygen consumption, % | 20 | 96.04 | 72.19 | 105.51 | 9.47 | 9.54 | 9.93 |
| MMb-reducing activity, % | 20 | 35.62 | 15.73 | 46.29 | 10.66 | 8.40 | 23.59 |
| Milligrams myoglobin/grams meat | 20 | 1.24 | 0.67 | 2.46 | 1.22 | 0.42 | 33.50 |
Standard deviation.
Coefficient of variation, calculated by (SD/mean) × 100%.
Chroma is a measure of color saturation, calculated by C = (a*2 + b*2)1/2.
Hue angle is a measure of the overall color of a sample, calculated by Hab = arctangent (b*/a*).
Calculated as .
Calculated as .
Calculated as % OMb = 100 − % MMb − % DMb.
Correlations of initial traits within muscle with final discoloration
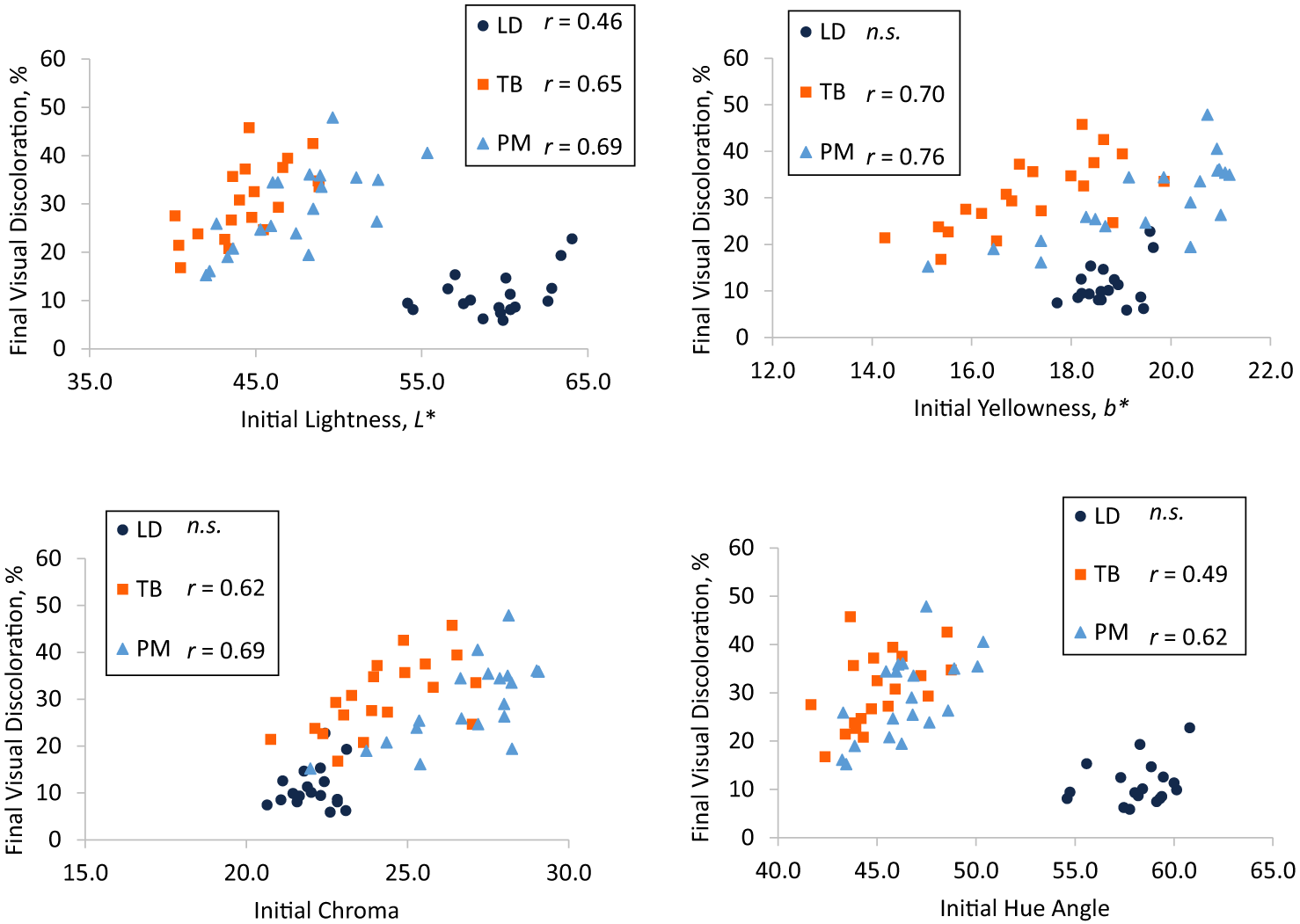
Despite averaging less than 2%, initial visual discoloration was correlated with final visual discoloration for LD (r = 0.76) but not for any other muscle (P ≥ 0.08; data not shown). Initial LD and TB lightness were moderately correlated to final LD and TB visual discoloration (r = 0.46 and r = 0.65, respectively), whereas initial PM lightness was strongly correlated to final PM visual discoloration (r = 0.69; Figure 1A). Correlation coefficients for initial L* and final visual discoloration did not differ (P ≥ 0.31) between muscles. Initial TB and PM yellowness were strongly correlated to final TB and PM visual discoloration (r = 0.70 and r = 0.76, respectively), but there was no correlation between initial LD yellowness and final LD visual discoloration (P = 0.11; Figure 1B). Initial TB chroma (r = 0.62; Figure 1C) and hue angle (r = 0.49; Figure 1D) were moderately correlated to final TB visual discoloration. Final PM visual discoloration was strongly correlated to initial PM chroma (r = 0.69; Figure 1C) and moderately correlated to initial PM hue angle (r = 0.62; Figure 1D). No correlations were observed between final LD visual discoloration and initial LD chroma or hue angle (P ≥ 0.32). Additionally, initial redness and 630/580 nm ratio were not correlated with final visual discoloration for any muscle (P ≥ 0.07). Correlation coefficients (r) did not differ (P ≥ 0.59) between TB and PM for the relationships between final visual discoloration and initial lightness, yellowness, chroma, or hue angle. In other words, the relationships between final visual discoloration and these traits were similar for TB and PM.
Pearson correlation coefficients between initial (day 1) (A) lightness, (B) yellowness, (C) chroma, and (D) hue angle and final (day 5) visual discoloration in longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM). Muscles with “n.s.” did not have significant correlations (P < 0.05). Correlations for muscles possessing different superscripts are different (P < 0.05).
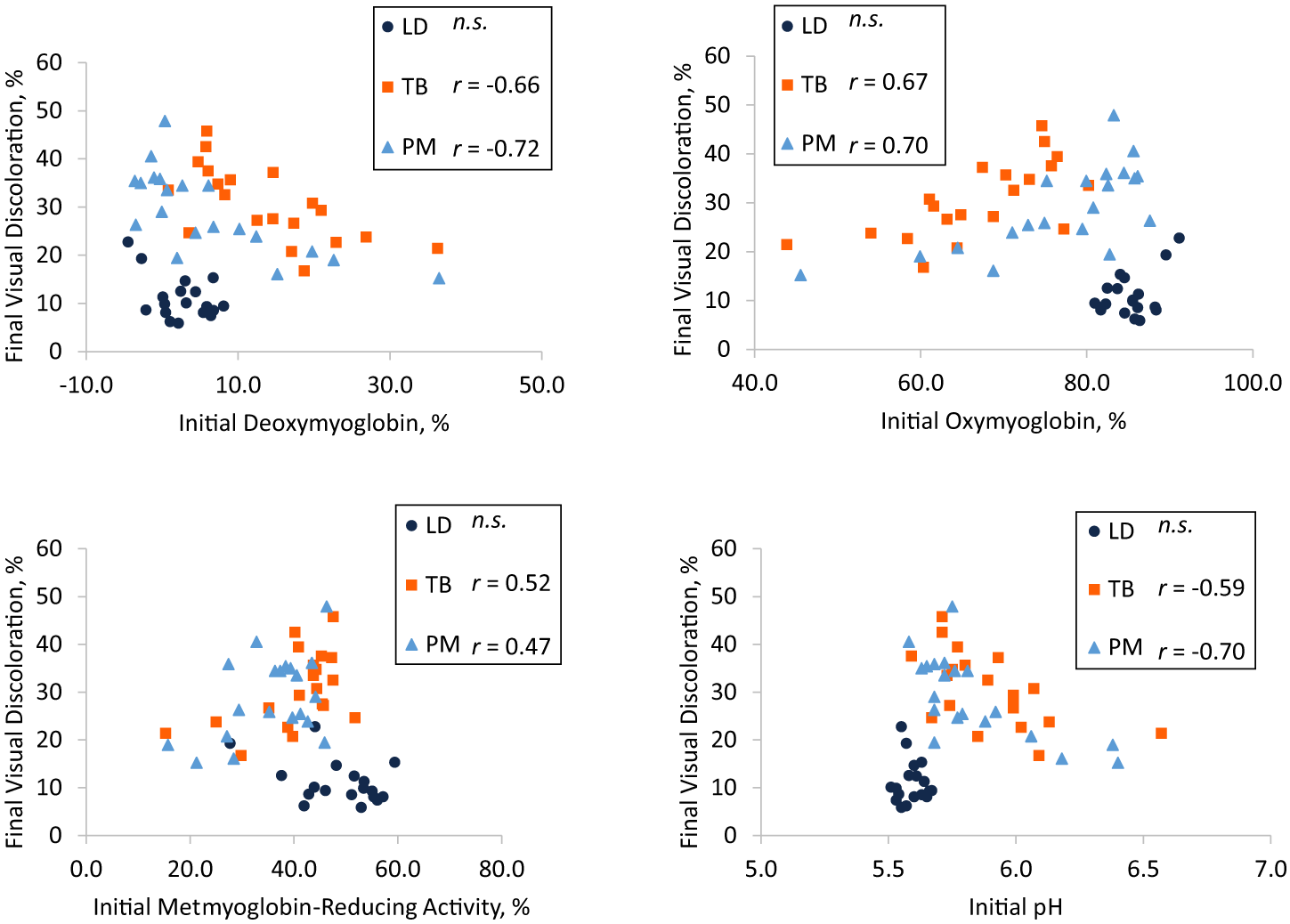
Final TB visual discoloration was moderately correlated to initial TB DMb (r = −0.66; Figure 2A), OMb (r = 0.67; Figure 2B), MRA (r = 0.52; Figure 2C), and pH (r = −0.59; Figure 2D). Final PM visual discoloration was moderately correlated to initial PM MRA (r = 0.47; Figure 2C) and strongly correlated to PM DMb (r = −0.72; Figure 2A), OMb (r = 0.70; Figure 2B), and pH (r = −0.70; Figure 2D). Initial OC was moderately correlated to final visual discoloration for TB (r = −0.48) but not correlated for LD or PM (P ≥ 0.06) (data not shown). Final LD visual discoloration was not correlated to initial LD OMb, DMb, MRA, or pH (P ≥ 0.06). No correlations were observed between final visual discoloration and initial Mb content for any muscle (P ≥ 0.09). Correlation coefficients (r) between final visual discoloration and initial OMb, DMb, MRA, or pH did not differ between TB and PM (P ≥ 0.59).
Pearson correlation coefficients between initial (day 1) (A) deoxymyoglobin, (B) oxymyoglobin, (C) metmyoglobin-reducing activity, and (D) pH and final (day 5) visual discoloration in longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM). Muscles with “n.s.” did not have significant correlations (P < 0.05). Correlations for muscles possessing different superscripts are different (P < 0.05).
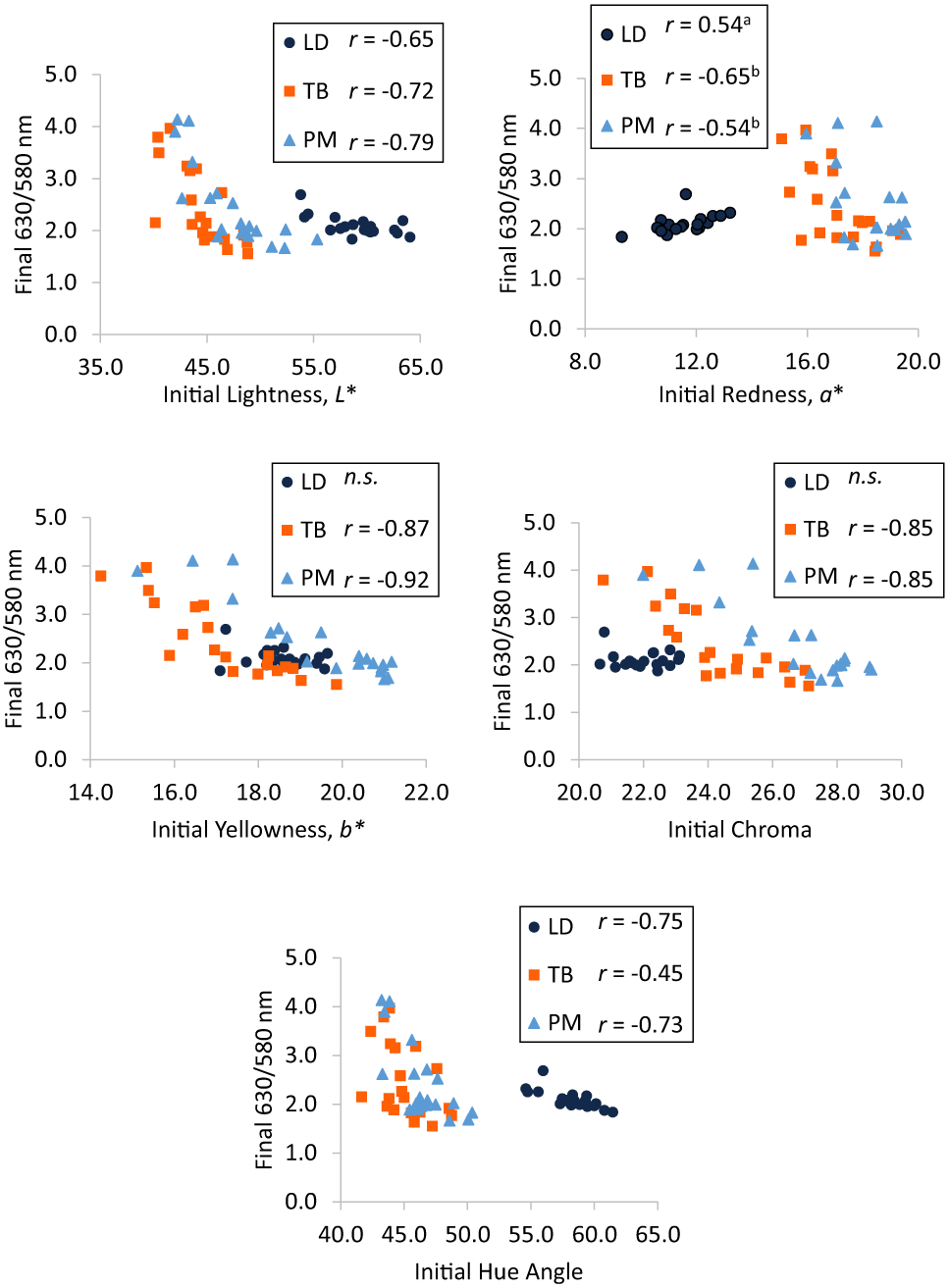
Final LD 630/580 nm ratio was moderately correlated to initial LD lightness (r = −0.65; Figure 3A) and redness (r = 0.54; Figure 3B) and strongly correlated to initial LD hue angle (r = −0.75; Figure 3E). Initial LD yellowness and chroma were not correlated to final LD 630/580 nm ratio (P ≥ 0.24). Final TB 630/580 nm ratio was moderately correlated to initial TB redness (r = −0.65; Figure 3B) and hue angle (r = −0.45; Figure 3E) and strongly correlated to initial TB lightness (r = −0.72; Figure 3A), yellowness (r = −0.87; Figure 3C), and chroma (r = −0.85; Figure 3D). Final PM 630/580 nm ratio was moderately correlated to initial PM redness (r = −0.54; Figure 3B) and strongly correlated to initial PM lightness (r = −0.79; Figure 3A), yellowness (r = −0.92; Figure 3C), chroma (r = −0.85; Figure 3D), and hue angle (r = −0.73; Figure 3E). Initial 630/580 nm ratio was strongly correlated to final visual discoloration for LD (r = 0.69) but not correlated for TB or PM (P ≥ 0.21; data not shown). Correlation coefficients between final 630/580 nm ratio and initial lightness or hue angle did not differ between any muscles (P ≥ 0.15). Correlations between final 630/580 nm ratio was positively correlated to initial redness in LD but negatively correlated to initial redness in TB and PM (P < 0.01). However, this same correlation did not differ between TB and PM (P = 0.64). Additionally, correlations between initial yellowness or chroma and final 630/580 nm ratio did not differ between TB and PM (P ≥ 0.46).
Pearson correlation coefficients between initial (day 1) (A) lightness, (B) redness, (C) yellowness, (D) chroma, and (E) hue angle and final (day 5) 630/580 nm ratio in longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM). Muscles with “n.s.” did not have significant correlations (P < 0.05). Correlations for muscles possessing different superscripts are different (P < 0.05).
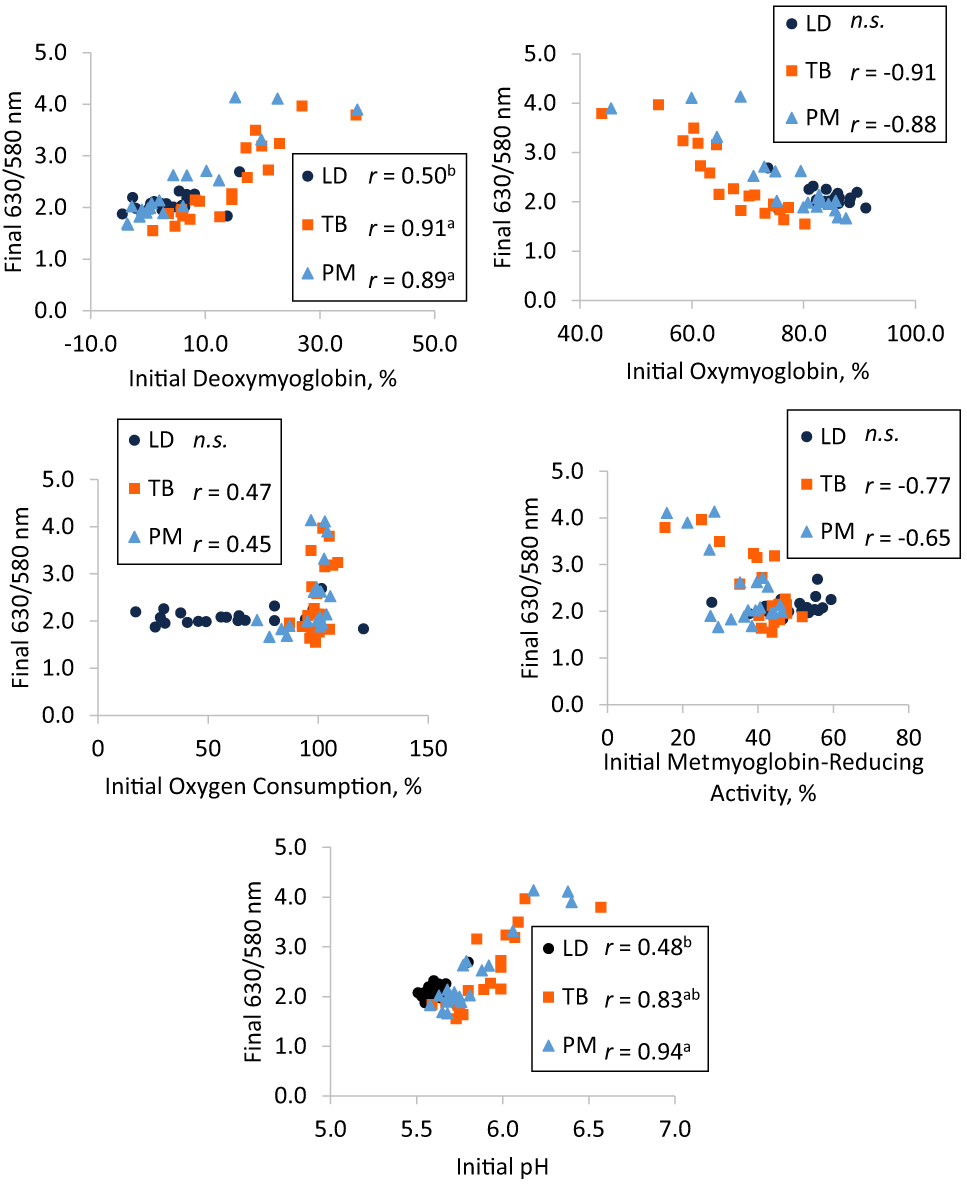
Final LD 630/580 nm ratio was moderately correlated to initial LD DMb (r = 0.50; Figure 4A) and pH (r = 0.48; Figure 4E). However, final LD 630/580 nm ratio was not correlated to initial LD OMb, OC, or MRA (P ≥ 0.32). Final TB 630/580 nm ratio was moderately correlated to initial TB OC (r = 0.47; Figure 4C) and strongly correlated to initial TB DMb (r = 0.91; Figure 4A), OMb (r = −0.91; Figure 4B), MRA (r = −0.77; Figure 4D), and pH (r = 0.83; Figure 4E). Final PM 630/580 nm ratio was moderately correlated to initial PM OC (r = 0.45; Figure 4C) and MRA (r = −0.65; Figure 4D) and strongly correlated to initial PM DMb (r = 0.89; Figure 4A), OMb (r = −0.88; Figure 4B), and pH (r = 0.94; Figure 4E). Initial MMb and Mb content were not correlated to final 630/580 nm ratio for any muscle (P ≥ 0.15). Correlations between final 630/580 ratio and initial DMb were stronger in TB and PM compared with LD (P < 0.01) but not different between TB and PM (P = 0.79). Correlations between initial pH and final 630/580 nm ratio were weaker in LD compared with PM (P < 0.01) but not different between LD and TB (P = 0.05) or between TB and PM (P = 0.10). There were also no differences between TB and PM for correlations between final 630/580 nm ratio and initial OMb, OC, or MRA (P ≥ 0.45).
Pearson correlation coefficients between initial (day 1) (A) deoxymyoglobin, (B) oxymyoglobin, (C) oxygen consumption, (D) metmyoglobin-reducing activity, and (E) pH and final (day 5) 630/580 nm ratio in longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM). Muscles with “n.s.” did not have significant correlations (P < 0.05). Correlations for muscles possessing different superscripts are different (P < 0.05).
Stepwise regression models
Models developed for visual discoloration explained 33% to 53% of variation for individual muscles (R2 = 0.33 to 0.53; P < 0.01; Table 2), whereas models developed for 630/580 nm ratio explained 60% to 85% of variation for individual muscle (R2 = 0.60 to 0.85; P < 0.01; Table 3). For both visual discoloration and 630/580 nm ratio, models for PM were most predictive (R2 = 0.53 and 0.85, respectively). Both final visual discoloration and final 630/580 nm ratio in TB (partial R2 = 0.49 and 0.76, respectively) and PM (partial R2 = 0.57 and 0.85, respectively) were primarily explained by initial yellowness. Furthermore, final LD 630/580 nm ratio was primarily explained by initial yellowness (partial R2 = 0.32) and redness (partial R2 = 0.29), with yellowness contributing slightly more to the model. Alternatively, final LD visual discoloration was primarily explained by initial lightness (partial R2 = 0.21) and Mb content (partial R2 = 0.20) and did not contain yellowness in the model. Despite explaining the majority of variation in the other models, yellowness was not included in the model for LD visual discoloration. Redness was included in visual discoloration models for LD and PM and 630/580 nm ratio models for PM but did not explain more than 2% of variation in any of those models. Ultimate pH, OC, MRA, and Mb content were also included in several of the models but explained no more than 5% (partial R2 = 0.05) of variation for any model and thus may not be practically important. Because of the low contributions of these traits to their respective models, the variation accounted for by these traits may not be practically important, and they may not be necessary in the models.
Stepwise regression models predicting final (day 5) visual discoloration (dependent variable) of longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) using initial (day 1) color and biochemical traits (independent variables)
| Initial (Day 1) Independent Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Item | Model R2 | Lightness, L* | Redness, a* | Yellowness, b* | Oxygen Consumption, % | Myoglobin, mg/g Meat | Intercept | Model P Value |
| LD | ||||||||
| R2 | 0.33 | 0.21 | — | — | — | 0.20 | — | <0.01 |
| — | 0.77 | — | — | — | 9.53 | −42.03 | — | |
| TB | ||||||||
| R2 | 0.48 | — | 0.02 | 0.49 | 0.02 | — | — | <0.01 |
| — | — | −2.13 | 3.98 | −0.54 | — | 52.75 | — | |
| PM | ||||||||
| R2 | 0.53 | — | 0.02 | 0.57 | — | 0.02 | — | <0.01 |
| β | — | — | −1.30 | 4.21 | — | 3.53 | −33.38 | — |
Stepwise regression models predicting final (day 5) 630/580 nm ratio (dependent variable) of longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) using initial (day 1) color and biochemical traits (independent variables)
| Initial (Day 1) Independent Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Model R2 | pH | Redness, a* | Yellowness, b* | Oxygen Consumption, % | Metmyoglobin-Reducing Activity, % | Myoglobin, mg/g Meat | Intercept | Model P Value |
| LD | |||||||||
| R2 | 0.60 | 0.05 | 0.29 | 0.32 | 0.03 | — | — | — | <0.01 |
| β | — | 0.92 | 0.16 | −0.13 | −0.001 | — | — | −2.29 | — |
| TB | |||||||||
| R2 | 0.78 | — | — | 0.76 | — | 0.05 | — | — | <0.0001 |
| β | — | — | — | −0.34 | — | −0.03 | — | 9.30 | — |
| PM | |||||||||
| R2 | 0.85 | — | 0.02 | 0.85 | 0.01 | 0.01 | 0.01 | — | <0.0001 |
| β | — | — | 0.17 | −0.48 | −0.01 | −0.01 | −0.39 | 10.88 | — |
Discussion
Differences in lightness of pork loins exhibiting quality conditions (pale, soft, and exudative [PSE]; normal; and dark, firm, and dry [DFD]) at day 1 of retail display were associated with final color stability after display (Zhu et al., 1998a). However, limited research has evaluated traits related to pork color stability in muscles other than LD and in the absence of obvious quality defects (PSE and DFD). Nearly 30% of the pork in the US is consumed as sausage or fresh pork from muscles other than LD or ribs (NPB, 2011). Although not all of this meat is overwrapped for retail sale, there is still potential for discounting or discarding of product because of discoloration. Therefore, there is value in identifying retail display traits related to discoloration in multiple pork muscles. For a predictive pork color stability system to be implemented, color traits determined at the production facility on the ventral surface of the loin (day 1 postmortem) must be related, with aged color traits determined on the loin chop face. Initially, however, it is important to assess whether aged loin chop color traits on entering the retail case are predictive of color stability during display.
Correlations between initial traits and final measures of discoloration were different between muscles but generally stronger for TB and PM than LD. Although correlations between initial traits and final 630/580 nm ratio were observed for all 3 muscles, correlations between initial traits and final 630/580 nm ratio for LD were numerically weaker for lightness, redness, DMb, and pH compared with correlations for TB and PM. For each of these initial traits except redness, LD traits were moderately correlated to the final 630/580 nm ratio, whereas TB and PM traits were strongly correlated. Furthermore, in several cases, initial LD traits were not correlated with discoloration measurements when the same traits were correlated in other muscles. For example, final visual discoloration was correlated to lightness, yellowness, chroma, hue angle, DMb, OMb, MRA, and pH in TB and PM chops but only correlated to lightness in LD chops. Similarly, the final 630/580 nm ratio was correlated to all day 1 traits except for the initial 630/580 nm ratio, initial MMb, and Mb in TB and PM. Alternatively, in LD, the 630/580 nm ratio was only correlated to lightness, redness, hue angle, DMb, and pH.
Differences in correlation strength may be attributable to variability differences in muscles. Smaller ranges in values and CV were observed for several LD traits compared with the same traits in PM and TB. For example, LD chops had lesser ranges and CV for pH, yellowness, chroma, hue angle, OMb, MRA, and Mb compared with TB and PM chops. Furthermore, LD chops discolored less overall compared with TB and PM chops, as evidenced by reduced visual discoloration scores on day 5 and smaller changes in the 630/580 nm ratio from day 1 to day 5. When variability among observations used to develop correlation coefficients is low, then the strength of the correlation itself will be decreased (Goodwin and Leach, 2006). This decrease in correlation strength is more apparent when observations for both the x and y variables have low variability (Goodwin and Leach, 2006). Thus, the lack of variation in initial traits and the lower overall extent of discoloration likely both resulted in a lack of correlations for LD. Reduced variability in loin traits could be expected. Pigs are commonly selected to minimize variation within LD because consumers prefer loin chops to be consistent when making purchasing decisions (King et al., 2011b). Alternatively, producers less commonly select pork to minimize variability in muscles other than the loin, resulting in more animal-to-animal variation for these muscles. For example, Barkley et al. (2021) reported that instrumental color was more variable in Boston butts (serratus ventralis muscle) than loins. Overall, the lack of variation in LD chops likely resulted in weaker relationships between initial retail display traits and final discoloration measures compared with TB and PM chops.
Despite differences in initial color and biochemical traits, minimal differences in correlation strengths were observed between TB and PM. No differences in correlation strength were observed between TB and PM when an initial trait was correlated to final visual discoloration or 630/580 nm ratio for both muscles. Studies evaluating discoloration differences between pork muscles are limited; however, multiple studies have characterized color stability in beef muscles. Both TB and PM are primarily oxidative muscles and have similar biochemical characteristics in beef (McKenna et al., 2005). In general, muscles characterized as “color labile” or “color stable” have similar characteristics to other muscles of the same category (Renerre and Labas, 1987; McKenna et al., 2005). Because of the similarities between PM and TB in beef, it was not unexpected that relationships between initial traits and discoloration would be similar between these 2 muscles in pork. Although further research is necessary to verify the color stability of other pork muscles, if relationships between the color-labile PM and TB were similar, then characteristics of these muscles may be indicative of color stability for other pork muscles that are considered color labile in beef, such as the supraspinatus, infraspinatus, and adductor (McKenna et al., 2005).
Correlations with initial traits were also stronger for the 630/580 nm ratio than visual discoloration for all muscles evaluated. Limited research has investigated differences in relationships between instrumental or visual measures of discoloration and color stability traits in pork or beef. However, instrumental measures are typically more precise than visual measures because they are evaluated using a machine (Barkley et al., 2018). Alternatively, because of the subjective nature of human sight, there is additional error associated with visual discoloration observations compared with instrumental assessment (Zhu and Brewer, 1999). Increased error for a set of measurements can also decrease correlation strength (Goodwin and Leach, 2006). As a result, relationships between initial traits and visual discoloration were weaker than relationships between the same initial traits and 630/580 nm ratio because of increased error associated with visual measurements. Therefore, measuring discoloration instrumentally may be more appropriate when evaluating relationships between discoloration and day 1 retail display traits.
The relationship between redness and discoloration was weaker than expected based on previous research in beef. Although redness was moderately correlated to final 630/580 ratio in all muscles, it was not correlated to any muscles for visual discoloration. Furthermore, redness partially explained the LD 630/580 nm ratio but was either not included in other muscles or explained very little variability (≤2%). This was unexpected because discoloration in beef is commonly associated with increased redness (McKenna et al., 2005; King et al., 2011a; Joseph et al., 2012; Canto et al., 2015), whereas neither lightness nor yellowness are commonly associated with color stability differences (McKenna et al., 2005; Canto et al., 2015). Increased redness in beef muscles is associated with a greater proportion of oxidative muscle fibers (Ramanathan et al., 2014). Oxidative muscle fibers contain greater levels of Mb, which is responsible for the red color of meat (King et al., 2023). However, in the current study, Mb content was not correlated to visual discoloration or 630/580 nm ratio for any muscle. Furthermore, although Mb content explained 20% of final LD visual discoloration variation in the current study, it did not explain more than 2% of variation in visual discoloration for TB or PM. Mb content did not explain any variation in the final 630/580 nm ratio for LD or TB and only explained 1% of variation for PM.
The lack of relationship between Mb content and discoloration traits in pork may stem from pork having less Mb than beef. Mb is present in smaller quantities in pork compared with beef. This is partially because pork is less oxidative than beef, with pork LD containing 10% Type I (red, oxidative fibers) and beef LD containing 30% Type I fibers (Zhang et al., 2017). In the current study, Mb content ranged from 0.74 (LD) to 2.01 (TB) mg/g on average compared with 4.05 to 4.56 mg/g for beef longissimus (Canto et al., 2015). The reduced Mb in pork may also contribute to the lack of relationship between redness and discoloration in this species.
Decreased lightness and yellowness were associated with more color-stable pork for all muscles evaluated. Final visual discoloration and 630/580 nm ratio were moderately or strongly correlated to lightness for all muscles and strongly correlated to yellowness for TB and PM. Furthermore, yellowness explained the majority of variation for visual and instrumental discoloration for all muscles evaluated in the study, with the exception of LD visual discoloration, which was explained by lightness and Mb. These results were in agreement with results from studies by King et al. (2011b) and Lindahl et al. (2006), who also reported that stable pork loins were darker and less yellow than labile loins. Decreases in both yellowness lightness in pork are associated with increased DMb content and decreased OMb content (Karamucki et al., 2013), both of which were also observed for stable pork in the current study. Overall, yellowness and lightness were the traits most strongly related to discoloration in muscles evaluated in this study, whereas redness was less important compared with beef.
Several studies conducted in beef have observed that labile beef possesses high OC rates, whereas stable beef has high MMb-reducing capacities (O’Keefe and Hood, 1982; Sammel et al., 2002; McKenna et al., 2005; Jeong et al., 2009), both across muscles and with a single muscle. In meat, mitochondria remain active postmortem and are able to consume oxygen (Mancini et al., 2018; Ramanathan and Mancini, 2018). When mitochondria consume oxygen, they provide electrons or reduced nicotinamide adenine dinucleotide for reduction of MMb, improving color stability (Ramanathan and Mancini, 2018). On its face, greater mitochondrial content, as evidenced by greater OC, would seem to favor improved color stability. However, mitochondrial degradation and loss of mitochondrial functionality places muscles under additional oxidative stress (Ramanathan and Mancini, 2018). Decreased color stability of beef PM was partially attributed to greater mitochondrial degeneration compared with LD (Mancini et al., 2018). Thus, greater OC in beef, although typically associated with higher mitochondria content, results in sharper color stability declines than when OC (and by extension, mitochondria content) is lower. Alternatively, MRA is typically increased in beef that is considered color stable (Reddy and Carpenter, 1991; Suman et al., 2014). Muscles with more glycolytic fibers have enzymes involved in anaerobic metabolism that are able to provide substrates for MMb reduction, minimizing MMb accumulation (Nair et al., 2018).
However, this relationship of OC and MRA with color stability did not hold true in pork PM and TB because samples with low visual discoloration and low 630/580 nm ratios displayed high OC and low MRA. As an example of related findings in pork, chops classified as DFD were more stable than normal pork chops and PSE pork chops over a 7 d display period (Zhu and Brewer, 1998b). However, different from beef, color-stable DFD chops also exhibited higher OC and lower MRA throughout display. In more color-stable DFD pork, whereas OC did decrease during storage, it decreased by a dramatically smaller magnitude compared with normal and PSE pork, which had smaller initial OC values (Zhu and Brewer, 1998b). Thus, it is interesting to speculate that differences in OC and MRA between the 2 species may be related to mitochondrial functionality. That said, OC of DFD pork may have also been favored by a higher pH compared with normal and PSE pork because mitochondrial OC increases at higher pH values (Ramanathan and Mancini, 2018). Nonetheless, in the present study, increased pH was also related to decreased visual discoloration and increased 630/580 nm ratio. This could indicate that mitochondria are more stable in pork compared with beef or that high pork pH favors OC enough to mitigate decreases in pork mitochondrial functionality. Furthermore, greater OC by mitochondria also limits the availability of oxygen for Mb interaction, minimizing the need for MMb to be reduced, which may explain lower MRA values in stable pork in the study by Zhu and Brewer (1998b) and the current study. This would also increase the proportion of DMb and decrease the proportion of OMb on the meat surface because of a lack of Mb oxygenation from lower oxygen levels, both of which were observed for stable samples in the current study. Further research is necessary to investigate differences in mechanisms behind OC and MRA between species. Nonetheless, results from the current study would indicate that stable pork possesses higher initial OC and DMb content and decreased initial MRA and OMb content.
Conclusions
Color and biochemical traits at the beginning of retail display were more strongly correlated with final visual discoloration and 630/580 nm ratio in PM and TB than LD. However, correlations did not differ in strength between PM and TB. Decreased discoloration and increased 630/580 nm ratio were associated with pork that was darker, less yellow, had decreased hue angles, and had increased DMb and pH values on day 1 of display for all muscles. In TB and PM, more color-stable pork was also associated with higher OC and lower MRA on day 1 of display. Initial yellowness was the best predictor of final visual discoloration for TB and PM and the best predictor of 630/580 nm ratio for all 3 muscles, whereas initial lightness and Mb content were the best predictors for final LD visual discoloration. Therefore, selection for pork with decreased lightness or yellowness in LD, TB, or PM could allow for increased pork color stability.
Literature Cited
Barkley, K. E., B. Fields, A. C. Dilger, and D. D. Boler. 2018. Rapid communication: Effect of machine, anatomical location, and replication on instrumental color of boneless pork loins. J. Anim. Sci. 96:2747–2752. doi: https://doi.org/10.1093/jas/sky223
Barkley, K. E., H. Rode, D. R. McKenna, D. D. Boler, B. N. Harsh, and A. C. Dilger. 2021. Effect of instrument settings and measurement environment on pork color measurements and variability. Meat Muscle Biol. 5:27, 1–15. doi: https://doi.org/10.22175/mmb.12245
Canto, A. C. V. C. S., S. P. Suman, M. N. Nair, S. Li, G. Rentfrow, C. M. Beach, T. J. P. Silva, T. L. Wheeler, S. D. Shackelford, A. Grayson, R. O. McKeith, and D. A. King. 2015. Differential abundance of sarcoplasmic proteome explains animal effect on beef Longissimus lumborum color stability. Meat Sci. 102:90–98. doi: https://doi.org/10.1016/j.meatsci.2014.11.011
Commission Internationale de l’Eclairage (CIE). 1976. Colorimetry – Part 4: CIE 1976 L*a*b* colour space. CIE Central Bureau, Vienna, Austria.
Cook, H., and L. Schulz. 2022. US pork industry: Current structure and economic importance. National Pork Producers Council. https://nppc.org/wp-content/uploads/2022/07/2021-NPPC-Economic-Contribution-Report-FINAL.pdf (Accessed 2 December 2022).
Faustman, C., and A. Phillips. 2001. Measurement of discoloration in fresh meat. Current Protocols in Food Analytical Chemistry. F3.3.1–F3.3.13. doi: https://doi.org/10.1002/0471142913.faf0303s00
Font-i-Furnols, M., and L. Guerrero. 2014. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 98:361–371. doi: https://doi.org/10.1016/j.meatsci.2014.06.025
Goodwin, L. D., and N. L. Leech. 2006. Understanding correlation: Factors that affect the size of r. J. Exp. Educ. 74:249–266. doi: https://doi.org/10.3200/JEXE.74.3.249-266
Jeong, J. Y., S. J. Hur, H. S. Yang, S. H. Mood, Y. H. Hwang, G. B. Park, and S. T. Joo. 2009. Discoloration characteristics of 3 major muscles from cattle during cold storage. J. Food Sci. 74:C1–C5. doi: https://doi.org/10.1111/j.1750-3841.2008.00983.x
Joseph, P., S. P. Suman, G. Rentfrow, S. Li, and C. M. Beach. 2012. Proteomics of muscle-specific beef color stability. J. Agr. Food Chem. 60:3196–3203. doi: https://doi.org/10.1021/jf204188v
Karamucki, T., M. Jakubowska, A. Rybarcyzk, and J. Gardzielewska. 2013. The influence of myoglobin on the colour of minced pork loin. Meat Sci. 94:234–238. doi: https://doi.org/10.1016/j.meatsci.2013.01.014
Kenny, D. A. 1987. Testing measures of association. In: Statistics for the social and behavioral sciences. Little, Brown and Company, Boston, MA. p. 270–291.
King, D. A., S. D. Shackelford, and T. L. Wheeler. 2011a. Relative contributions of animal and muscle effects to variation in beef lean color stability. J. Anim. Sci. 89:1434–1451. doi: https://doi.org/10.2527/jas.2010-3595
King, D. A., S. D. Shackelford, and T. L. Wheeler. 2011b. Use of visible and near-infrared spectroscopy to predict pork longissimus lean color stability. J. Anim. Sci. 89:4195–4206. doi: https://doi.org/10.2527/jas.2011-4132
King, D. A., M. C. Hunt, S. Barbut, J. R. Claus, D. P. Cornforth, P. Joseph, Y. H. B. Kim, G. Lindahl, R. A. Mancini, M. N. Nair, K. J. Merok, A. Milkowski, A. Mohan, F. Pohlman, R. Ramanathan, C. R. Raines, M. Seyfert, O. Sørheim, S. P. Suman, and M. Weber. 2023. American Meat Science Association guidelines for meat color measurement. Meat Muscle Biol. 6:12473, 1–81. doi: https://doi.org/10.22175/mmb.12473
Krzywicki, K. 1979. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. doi: https://doi.org/10.1016/0309-1740(79)90019-6
Lindahl, G., A. H. Karlsson, K. Lundström, and H. J. Andersen. 2006. Significance of storage time on degree of blooming and colour stability of pork loin from different crossbreeds. Meat Sci. 72:603–612. doi: https://doi.org/10.1016/j.meatsci.2005.09.018
Livers, J. J. 1942. Some limitations to use of coefficient of variation. J. Farm Econ. 24:892–895.
Lowell, J. E., M. F. Overholt, B. N. Harsh, C. A. Stahl, A. C. Dilger, and D. D. Boler. 2017. Relationships among early postmortem loin quality and aged loin and pork quality characteristics between barrows and gilts. Translational Animal Science 1:607–619. doi: https://doi.org/10.2527/tas2017.0074
Mancini, R. A., K. Belskie, S. P. Suman, and R. Ramanathan. 2018. Muscle-specific mitochondrial functionality and its influence on fresh beef color stability. J. Food Sci. 83:2077–2082. doi: https://doi.org/10.1111/1750-3841.14219
McKenna, D. R., P. D. Mies, B. E. Baird, K. D. Pfeiffer, J. W. Ellebracht, and J. W. Savell. 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682. doi: https://doi.org/10.1016/j.meatsci.2005.02.016
Mills, B. I. 2021. 2015 and 2018 national meat case studies: Packaging type, marketing claims, and allocation in the self-service meat case. M.S. thesis, Texas Tech Univ., Lubbock, TX. (https://hdl.handle.net/2346/87855)
Nair, M. N., S. Li, C. M. Beach, G. Rentfrow, and S. P. Suman. 2018. Changes in the sarcoplasmic proteome of beef muscles with differential color stability during postmortem aging. Meat Muscle Biol. 2:1–17. doi: https://doi.org/10.22175/mmb2017.07.0037
National Pork Board (NPB). 2011. Quick facts: The pork industry at a glance. Pork Checkoff, National Pork Board, Des Moines, IA.
O’Keefe, M., and D. E. Hood. 1982. Biochemical factors influencing metmyoglobin formation on beef from muscles of differing colour stability. Meat Sci. 7:209–228. doi: https://doi.org/10.1016/0309-1740(82)90087-0
Raines, C. R., M. C. Hunt, and J. A. Unruh. 2010. Contributions of muscles of various color stabilities to the overall color stability of ground beef. J. Food Sci. 75:C85–C89. doi: https://doi.org/10.1111/j.1750-3841.2009.01430.x
Ramanathan, R., S. P. Suman, and C. Faustman. 2014. Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: A review. J. Agr. Food Chem. 68:12779–12787. doi: https://doi.org/10.1021/acs.jafc.9b08098
Ramanathan, R., and R. A. Mancini. 2018. Role of mitochondria in beef color: A review. Meat Muscle Biol. 2:309–320. doi: https://doi.org/10.22175/mmb2018.05.0013
Ramanathan, R., M. C. Hunt, T. Price, and G. G. Mafi. 2021. Chapter five - Strategies to limit meat wastage: Focus on meat discoloration. Advances in Food and Nutrition Research. 95:183–205. doi: https://doi.org/10.1016/bs.afnr.2020.08.002
Reddy, L. M., and C. E. Carpenter. 1991. Determination of metmyoglobin reductase activity in bovine skeletal muscles. J. Food Sci. 56:1161–1164. doi: https://doi.org/10.1111/j.1365-2621.1991.tb04724.x
Renerre, M., and R. Labas. 1987. Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Sci. 19:151–165. doi: https://doi.org/10.1016/0309-1740(87)90020-9
Sammel, L. M., M. C. Hunt, D. H. Kropf, K. A. Hachmeister, and D. E. Johnson. 2002. Comparison of assays for metmyoglobin reducing activity in beef inside and outside semimembranosus muscle. J. Food Sci. 67:978–984. doi: https://doi.org/10.1111/j.1365-2621.2002.tb09439.x
Suman, S. P., M. C. Hunt, M. N. Nair, and G. Rentfrow. 2014. Improving beef color stability: Practical strategies and underlying mechanisms. Meat Sci. 98:490–504. doi: https://doi.org/10.1016/j.meatsci.2014.06.032
US Department of Agriculture (USDA). 2021. Statistics & information. https://www.ers.usda.gov/topics/animal-products/cattle-beef/statistics-information.aspx. (Accessed 9 September 2021.)
Zhang, X., C. M. Owens, and M. W. Schilling. 2017. Meat: The edible flesh from mammals only or does it include poultry, fish, and seafood? Animal Frontiers. 7:12–18. doi: https://doi.org/10.2527/af.2017.0437
Zhu, L. G., and M. S. Brewer. 1998a. Discoloration of fresh pork as related to muscle and display conditions. J. Food Sci. 63:763–767. doi: https://doi.org/10.1111/j.1365-2621.1998.tb17895.x
Zhu, L. G., and M. S. Brewer. 1998b. Metmyoglobin reducing capacity of fresh normal, PSE, and DFD pork during retail display. J. Food Sci. 63:390–390. doi: https://doi.org/10.1111/j.1365-2621.1998.tb15749.x
Zhu, L. G., and M. S. Brewer. 1999. Relationship between instrumental and visual color in a raw, fresh beef and chicken model system. J. Muscle Foods. 10:131–146. doi: https://doi.org/10.1111/j.1745-4573.1999.tb00391.x