Introduction
Meat spoilage is a serious problem that affects various parts of the food chain including production, storage, and consumption. It makes meat products undesirable for consumption and results in the decrease of shelf life and meat quality because of undesirable changes in physical and chemical characteristics such as the formation of off-flavors, discoloration, off-odors, and slime (Pellissery et al., 2020). Spoilage is a process involving various bacteria species and environmental factors such as the composition of the product itself, storage temperature, and product pH (Anas et al., 2019). The nutrition-rich composition of meat and meat products further supports the microbial spoilage process, allowing the establishment of the microbial community (Anas et al., 2019). If the richness and abundance of spoilage-related species in this community reach an unacceptable level, it negatively impacts shelf life and even microbial safety of the product (Zhao et al., 2015). Because of microbial spoilage, lipid oxidation, and discoloration, excessive amounts of meat products have been wasted in the meat industry supply chain or discarded by customers (Falowo et al., 2014; Karwowska et al., 2021). Meat processing establishments want to minimize and ideally eliminate microbial contamination to reduce industrial and economic losses and minimize or prevent meat waste as well as to keep their products on the shelves longer without a reduction in quality (Sofos, 2014; Berry, 2019). Concomitantly, consumers expect to access meat products that are wholesome, free of contamination, nutritious, and easy to prepare (e.g., ready-to-eat [RTE]). RTE meat products are especially preferred because of consumers’ busier lifestyles; however, they are also highly open to increasing opportunities for contamination (USDA, 2016). In general, properly cooked RTE meat and poultry products are expected to be free of microbiological contamination after thermal processes. However, many postcooking handling steps increase the risk of microbial contamination, which can be traced back to workers, environment, equipment, and processes such as packaging and slicing (Dempster et al., 1973; Korkeala and Björkroth, 1997; Samelis et al., 2000a, 2000b; Pothakos et al., 2015; Odeyemi et al., 2020). Even though RTE meats are processed in highly hygienic conditions and typically begin with an undetectable bacteria level (under 10 CFU/g) at the beginning of shelf life, spoilage can still occur quickly (Hamasaki et al., 2003).
The spoilage of carbon dioxide–modified atmosphere or vacuum-packaged RTE meat products is primarily caused by lactic acid bacteria (LAB) and is considered an economic loss rather than a food safety concern (Sarmento et al., 2015; Iulietto et al., 2015). Lactobacillus, Leuconostoc, Enterococcus, Lactococcus, and Streptococcus have been demonstrated to be the predominant flora in vacuum-packaged cooked and refrigerated meat products (Chenoll et al., 2007; Chávez-Martinez et al., 2016). These LAB detected in the high-hygiene packaging area have been reported to constitute the major microbiota of the cooked sausages (Hultman et al., 2015). These researchers further identified the LAB species as Leuconostoc mesenteroides, Leuconostoc pseudomesenteroides, Leuconostoc gelidum subsp. gasicomitatum, Lactobacillus curvatus, and Lactococcus lactis. In the microbiota of RTE meat products, Lactobacillus sakei and Leuconostoc mesenteroides have been regularly reported as the main predominant species (Dykes et al., 1994; Yang and Ray, 1994; Samelis et al., 1998, 2000a, 2000b). L. lactis and Enterococcus faecalis have also been found to be part of the predominant microbiota of RTE meat products (Barakat et al., 2000). Furthermore, it is known that nitrite inhibits the growth of some gram-negative microorganisms (Borch et al., 1996). Therefore, nitrite contributes to the proliferation of gram-positive microorganisms such as LAB in cured and processed meats.
The application of organic acids and their salts is one of the most common and effectively used methods for controlling pathogenic bacteria in post-thermal process contamination in RTE meat products. (Glass et al., 2002; Mbandi and Shelef, 2002; Samelis et al., 2005; Maks et al., 2010; Stopforth et al., 2010). However, limited studies are available on RTE meat products to reveal the effect of organic acid salts on the mixture of spoilage LAB. Drosinos et al. (2006) applied different organic acid salts (sodium lactate [SL], sodium acetate, potassium sorbate, and their combinations) in different concentrations to both broth system and cooked vacuum-packaged RTE meat products stored for 40 d at 4°C. They reported an antimicrobial effect on the growth of spoilage LAB with the increase in concentration. Similar results were reached with SL regarding LAB in cooked, vacuum-packaged ham stored at similar conditions (Stekelenburg and Kant-Muermans, 2001). It has also been reported that shelf life doubled when the SL concentration increased from 2.5% to 3.3% (Stekelenburg and Kant-Muermans, 2001). These types of studies are valuable to serve as fundamental investigations to the elude the effect of organic acids on spoilage LAB; however, they were limited to only bacterial colony enumeration in microbiological challenging tests and were not able to demonstrate how microbial community structure changed during storage.
Most recently, 16S rRNA gene sequencing has enabled the characterization of microbial diversity and relative abundances in meat systems revealing microbial community dynamics during storage time (Ercolini, 2013; Rouger et al., 2017). It is important to evaluate microbiota dynamics because each bacterial group may contribute to meat spoilage; however, spoilage contribution might change depending on environment and microbial community structure (Zhao et al., 2015; Cauchie et al., 2020). Therefore, a strong knowledge of microbial community composition and its dynamics under certain circumstances is crucial for the elimination or control of spoilage microorganisms in the preservation of meat products. Importantly, the behavior of LAB species in microbial communities and their interactions with their environment are not well understood, especially in RTE meat products. Consequently, more information is needed about how the spoilage communities change throughout storage with the application of different antimicrobials to improve the microbiological safety and the shelf life of RTE meat products. According to our knowledge, there is no study specifically focused on the effect of different antimicrobials on inoculated spoilage microbiota of vacuum-packaged, cooked, and cold-stored RTE meat products. Therefore, the objective of this study was to investigate how the most commonly used antimicrobials (sodium diacetate [SD], and the combination of SL and SD) affect the microbial community structure of spoilage LAB in deli-style turkey breast during 35 d of storage when applied at similar concentrations as used for controlling Listeria monocytogenes.
Material and Methods
Product manufacture
Cured deli-style turkey breasts were manufactured following good manufacturing practices at the University of Wisconsin-Madison, Meat Science and Muscle Biology Laboratory. Frozen, whole, skinless, and boneless turkey breasts were purchased from local suppliers and stored as frozen (−25°C) until needed. After thawing at 2.2°C to 4.4°C, the breasts were ground using a grinder (Hobart Model 4732, Hobart Corporation, Troy, OH) to 9.53 mm.
Product formulations for microbiological analysis were targeted to achieve a 100% cook yield (no-cook loss in moisture proof plastic casing) after thermal processing. The main formulation (1.7% salt, 0.30% sodium tripolyphosphate [STPP], 1.0% modified food starch, 100 mg/kg sodium nitrite, and 547 mg/kg sodium erythorbate) was used in all treatments to mimic typical formulations used in the industry for a cured deli-style turkey breast. Cured deli-style turkey breast formulations, with an adjusted pH of 6.3 and moisture of 75.0%, were prepared with 0.125% SD (Sigma-Aldrich, St. Louis, MO), and SD + 2.5% SL (syrup, 60%) (w/w) (Sigma-Aldrich) as well as a control containing no antimicrobial for the current microbiota study. Each treatment was manufactured in 3 replications on 3 different days of production to demonstrate different raw turkey breast batches.
The details of treatment formulations were calculated to approximate moisture levels of turkey meat and all added ingredients, whereas the required ingoing levels of each formulated ingredient served as limitations (i.e., 1.7% salt, 0.30% STPP, 1.0% modified food starch, 100 mg/kg sodium nitrite, and 547 mg/kg sodium erythorbate were constraints for final product levels in all formulations).
Ingredients for all formulations were dissolved in water to provide uniform ingredient distribution. The ingredients were added into a solution in the following order: STPP, salt, sodium nitrite, sodium erythorbate, antimicrobials, sodium bicarbonate (if necessary), and modified food starch. After combining the ingredients, the solution was mixed with ground turkey meat for 2 min in a stand mixer (Hobart A120, Hobart Corporation). The mixture was then transferred to a rotary vane vacuum filler (Handtmann VF 608 Plus vacuum filler, Handtmann CNC Technologies Inc., Lake Forest, IL) and stuffed into (90 mm flat width) moisture impermeable plastic casings (Nova X, ViskoTeepak USA, Kenosha, WI) to produce chubs that were approximately 65 cm in length. A clipper (Poly-clip model EZ 6080, Poly-clip System Corp., Mundelein, IL) was used to close tightly the ends of each chub.
Cooking was accomplished using a steam-jacketed kettle (Groen model N30, Groen Mfg. Co., Chicago, IL) preheated to 80°C until the internal temperature of the chubs reached 73.9°C. After cooking, products were immediately chilled in icy water for 20 min and then placed in a cooler (∼4°C) until they were sliced the following day.
All cooked turkey chubs were sliced using a manual deli slicer (Berkel Model 919E, Berkel Incorporated, Troy, OH). To minimize contamination risk with background microflora, a 70:30 ethanol/distilled water solution was sprayed on all product contact surface areas and the exterior of the plastic casings of each treatment chub prior to slicing. The peeling process for each chub was done immediately before slicing to reduce the possibility of recontamination. The products were sliced to a target thickness of 25 ± 1 g per slice. All treatments were immediately vacuum sealed (45.7 × 71.1 cm, 3-mil high-barrier pouches; UltraSource LLC, Kansas City, MO) after slicing and transferred to the Food Research Institute at the University of Wisconsin-Madison for microbial inoculation and sample collection. The process started 1 to 2 d after slicing, and the vacuum-sealed master packs of the sliced samples were stored at 4°C until inoculation.
Microbial inoculation and sample collection
Following 3 replications per treatment, duplicate samples (each containing two 25 g slices) per treatment were inoculated with a mixture of 5 LAB strains. Two different strains of L. mesenteroides (bacon and deli-shaved ham isolates), L. sakei, L. lactis (meat isolate), and E. faecium (meat isolate) originating from 2 different commercial meat-related companies were grown individually in 9 mL All Purpose Tween (APT) broth (BD Difco, Sparks, MD) at 30°C for 20 to 22 h. LAB species were harvested by centrifugation (4,000 × g, 20 min) and suspended in 4.5 mL of Butterfield’s phosphate buffer. Equivalent populations of each strain were combined to obtain a 5-strain blend of LAB for the inoculation of products. Each LAB strain population and their mixtures were validated by plating on APT agar (BD Difco). The 2 turkey breast slices per package were surface inoculated with a target of 3 log CFU/g of 5 different LAB strain blends (5 log CFU/50 g package) in total by applying 0.25 mL inoculum onto various surface areas. Both inoculated and uninoculated slices were placed in gas-impermeable vacuum chamber pouches (3 mil, 7 × 9”, UltraSource LLC) and vacuum-packaged (Multivac AGW, Sepp Haggenmueller KG, Wolfertschwenden, Germany) prior to being stored at 4°C for up to 42 d.
Rinse material from each sample for microbiota analysis was collected on days 0 (inoculation day), 7, 14, 21, 35, and 42 by washing and hand massaging for 3 min with 50 mL of sterile Butterfield’s phosphate buffer solution. One and a half milliliters of rinsate from each technical replicate of inoculated and uninoculated samples was collected into 1.5 mL Eppendorf tubes and stored at −80°C for later microbiota analysis. For this microbiota study, the effect of control, SD, and the combination of SL and SD were tested from the samples collected at days 0, 7, 14, 21, and 35.
DNA extraction, polymerase chain reaction, and library preparation
Frozen samples (−80°C) were thawed on ice for the DNA extraction process applying a modified mechanical cellular disruption method and hot/cold phenol (Stevenson and Weimer, 2007) method. Briefly, bead-beating tubes with 0.5 g of 0.1 mm beads were filled with 1 mL of the sample, 700 μL equilibrated phenol, and 50 μL 20% sodium dodecyl sulfate and were bead-beaten for 2 min. Following incubation in a water bath at 60°C for 10 min, the tubes were bead-beaten for an additional 2 min followed by centrifuging (Hermle Benchmark - Z 216 MK, HERMLE Labortechnik GmbH, Wehingen, Germany) (20,000 × g, 4°C, 10 min). The aqueous phase of samples was carefully transferred into phenol-safe 1.5 mL Eppendorf tubes, and DNAs of the samples were extracted with 500 μL phenol:chloroform:isoamyl alcohol 25:24:1. Tubes were briefly vortexed and centrifuged (20,000 × g, 4°C, 10 min). The previous phenol:chloroform:isoamyl alcohol wash step was then repeated to get the DNAs as pure as possible, followed by vortexing and centrifugation.
The aqueous phase of samples was collected into new tubes containing 0.1 vol 2M sodium acetate and 0.6 vol (including acetate) isopropyl alcohol. Tubes were mixed by inversion and stored at −20°C overnight for DNA precipitation. After the precipitation process, samples were centrifuged (20,000 × g, 4°C, 20 min) and the supernatants were discarded. Pellets were carefully washed with 1 mL 70% ethanol and centrifuged (20,000 × g, 4°C, 1 min). The ethanol wash step was repeated using 500 μL ethanol followed by additionally centrifuging (20,000 × g, 4°C, 3 min) followed by air drying in the fume hood until no ethanol remained. The dried pellets were suspended in 40 μL of Zymogen DNA elution buffer (Zymo Research, Irvine, CA; D3004-4).
The DNA concentrations of the samples were determined using the Qubit dsDNA HS (High Sensitivity) Assay Kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA) by a Qubit Fluorometer (Invitrogen, San Diego, CA).
The V4 variable region of the 16S rRNA gene was amplified using barcoded universal bacterial primers (F-GTGCCAGCMGCCGCGGTAA; R-GGACTACHVGGGTWTCTAAT) demonstrated by Kozich et al. (2013). The primers also contained adapters suitable for sequencing on Illumina platforms (F-AATGATACGGCGACCACCGAGATCTACAC; R-CAAGCAGAAGACGGCATACGAGAT). Polymerase chain reaction (PCR) reactions were performed in 20 μL reaction mixtures containing 12.5 μL 1X Terra PCR Direct Buffer (Clontech Laboratories Inc., Mountain View, CA), 0.6 μL Terra PCR Direct Polymerase Mix (Clontech Laboratories Inc.), 10 ng template DNA, 0.75 μL forward and reverse primers (10 μM/μL), and water to a total volume. Nuclease-free water was used as a no-template PCR negative control.
A Bio-Rad S1000 thermal cycler (Bio-Rad Laboratories, Hercules, CA) was used for PCR reactions following protocol: initial denaturation at 98°C for 2 min, followed by 30 cycles of 98°C for 30 s, 62°C for 1 min, and 68°C for 45 s, and a final extension of 68°C for 5 min. After amplification, PCR products were run on a 1.0% (wt/vol) low melt agarose gel (Invitrogen). Amplicon bands at ∼380 bp were cut and purified using a ZR 96 Zymoclean Gel DNA Recovery Kit (Zymo Research). Purified products were then quantified using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific) and a Synergy 2 Multi-Mode plate reader (BioTek, Winooski, VT) and were equimolar pooled into a 4 nM library. The final library was sequenced on an Illumina MiSeq using a 500-cycle (2 × 250 bp) PE MiSeq v2 reagent kit (Illumina, San Diego, CA) with a 10% PhiX control and custom sequencing primers (Kozich et al., 2013) at the University of Wisconsin-Madison Biotechnology Center.
Sequence analysis
Sequences were demultiplexed on the Illumina MiSeq and were further processed using mothur v.1.44.3 (Schloss et al., 2009) following a protocol developed by Kozich et al. (2013). In short, sequences with ambiguous base pairs, homopolymers greater than 8 bp, and lengths shorter than 200 bp and greater than 500 bp were removed. Sequences were aligned against the SILVA 16S rRNA gene database v138 (Pruesse et al., 2007) and those not aligning to the V4 region were removed. Preclustering was performed (diffs = 2) for error reduction and chimeras were detected and removed (Edgar et al., 2011). Sequences were then taxonomically classified using the mothur-formatted SILVA taxonomic database v. 138 with a bootstrap cutoff of 80. Unclassified sequences and sequences classified as mitochondria, chloroplasts, Eukarya, or Archaea were removed. Singletons were also removed. Sequences were then grouped into operational taxonomic units (OTUs) at 97% sequence similarity. Good’s coverage (Good, 1953) was calculated, and samples were normalized to 489 sequences per sample. Chao1 richness (Chao, 1984) and Shannon’s diversity index (Shannon, 1948) were then estimated for each normalized sample using mothur. OTUs were classified using SILVA taxonomic database v. 138 for final classification.
Statistical analysis
The OTU table was subset to include only OTUs classified as the order “Lactobacillales” for subsequent analyses because of our specific interest in the community structure of the inoculated 5 different spoilage LAB species. Of the 289 total OTUs in the dataset, 30 were classified as Lactobacillales and constituted our final dataset for subsequent analysis. Statistical analyses were performed in R (v. 4.0.2; R Core Team, 2020).
For α-diversity, Chao1 richness (number of species) and Shannon’s diversity index (a measure of how evenly microbes are distributed) estimates were used. The α statistics were calculated using vegan and phyloseq packages in R (McMurdie and Holmes, 2013). Before running statistical analyses, the Shapiro-Wilk test was used to evaluate the normality of Chao1 (W = 0.63, P = 1.752e-09) and Shannon (W = 0.67, P = 9.92e-09) indexes. Being non-normally distributed, the significance of the differences between treatment groups was assessed using the Kruskal-Wallis test for Chao1 and Shannon’s diversity. Furthermore, the paired Wilcoxon rank-sum test was used with false discovery rate (FDR)-corrected P values to determine which groups were different.
For β-diversity, it is hard to test the microbiota composition directly using OTUs or abundances because of the high dimensionality and phylogenetic structure of the microbiota data. The significance of separation in β-diversity space was assessed by permutational multivariate analysis of variance (PERMANOVA). Variance homogeneity assumption for PERMANOVA, which is similar dispersion within each group, was tested applying “betadisper” and then “permutes” in R “vegan” package (Oksanen et al., 2019). The Bray-Curtis dissimilarity (diversity based on abundance; community structure) and Jaccard (richness based on OTU presence-absence; community composition) distance metrics were calculated beforehand (metaMDS; vegan). Data were analyzed as 3 treatments (C, SD, SL + SD [SLSD]) by 5 time points (day: 0, 7, 14, 21, 35), including interaction with the day as a repeated measure. PERMANOVA test was stratified by sampling day with strata argument to account for external variability between treatment groups to see if treatments differ while controlling for time (adonis; vegan package). The null hypothesis (H0) was microbiota composition is the same across sampling days, whereas the alternative hypothesis (H1) was microbiota composition differs between sampling days. FDR-corrected P values were calculated using “vegdist” (vegan package). A nonmetric multidimensional scaling (NMDS) plot was used to visualize β-diversity. Community taxonomy composition, β-diversity, and α-diversity metrics were visualized using the R package ggplot2 (Wickham, 2009) and phyloseq. Significance was established at P < 0.05.
Results
Sequencing generated 4,308,005 raw sequences, of which 2,752,303 remained after the cleanup process. All samples had Good’s coverage >0.95. The number of sequences in each sample ranged from 489 to 128,482 with a mean of 60,246 and a median of 80,623 sequences. When separated by treatment, control with no antimicrobial ranged from 575 to 102,809 sequences, diacetate ranged from 506 to 126,255, and the combination of lactate-diacetate ranged from 489 to 128,482 sequences.
α-diversity
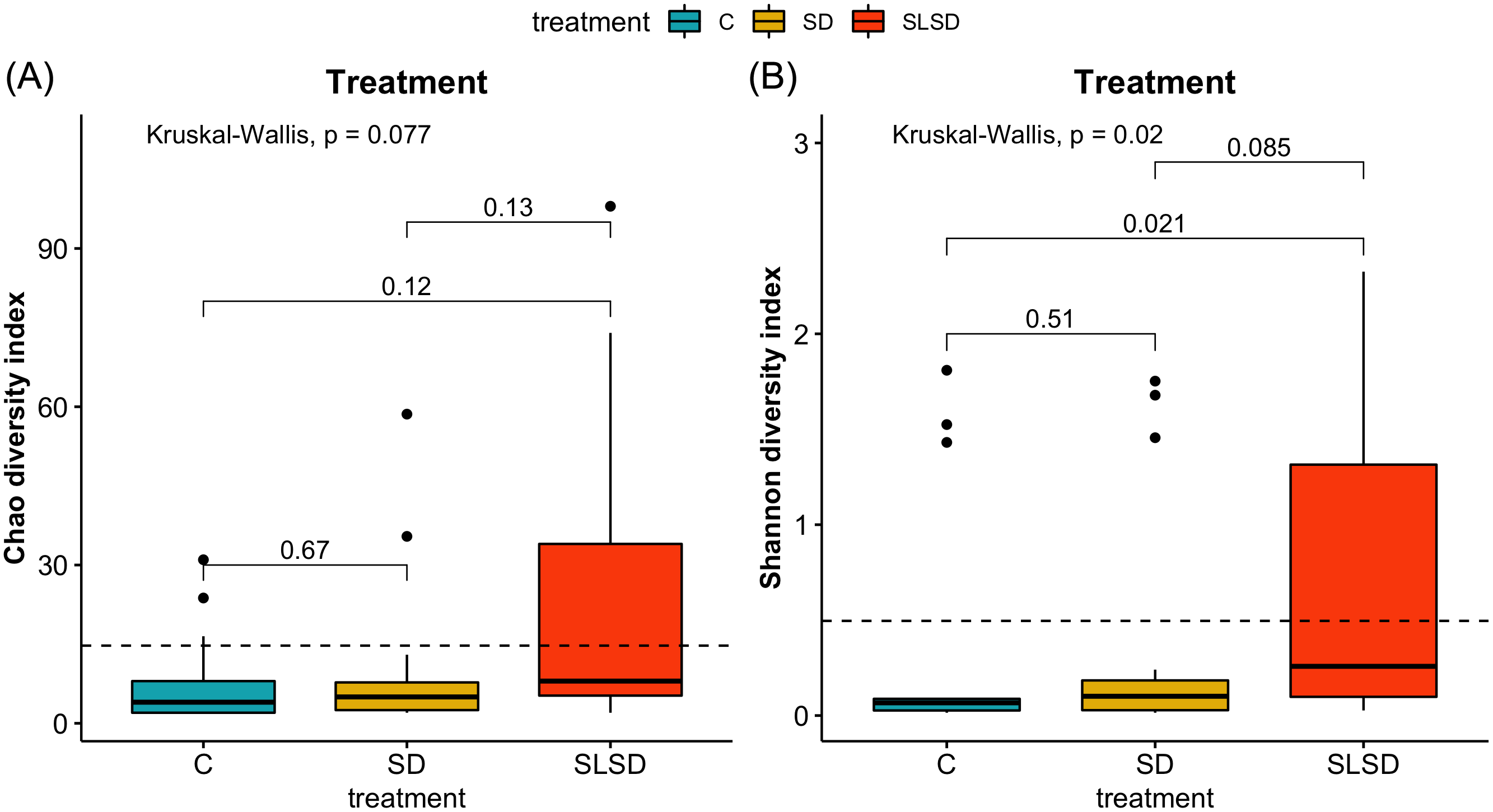
Shapiro-Wilk normality test showed that the α-diversity metrics, Chao1 and Shannon, did not have normal distributions (P < 0.001). Therefore, the Kruskal-Wallis rank-sum test was used to test differences of the Chao1 richness estimate and Shannon’s diversity metrics. No significant differences were found between replicates (P = 0.217). Both Chao1 and Shannon demonstrated reduced bacterial richness and diversity as the storage period increased. The differences between treatments for Chao1 were not significant in the Kruskal-Wallis rank-sum test (P = 0.077) (Figure 1), whereas it was significant for Shannon (P = 0.020) (Figure 1). Furthermore, the paired Wilcoxon rank-sum test applied on Shannon and revealed that SLSD treatment had a significantly higher bacterial diversity compared with C (P = 0.021) (Figure 1), whereas SD did not show any significant difference from C (P = 0.51) and SLSD (P = 0.085) (Figure 1).
α-diversity comparisons. Richness estimators: (A) Chao1 diversity index and evenness estimator; (B) Shannon’s diversity index. P values for pairwise comparison of the treatments obtained with the pairwise Wilcox rank-sum test (false discovery rate, 0.05). C = control; SD = sodium diacetate; SLSD = sodium lactate + sodium diacetate.
β-diversity
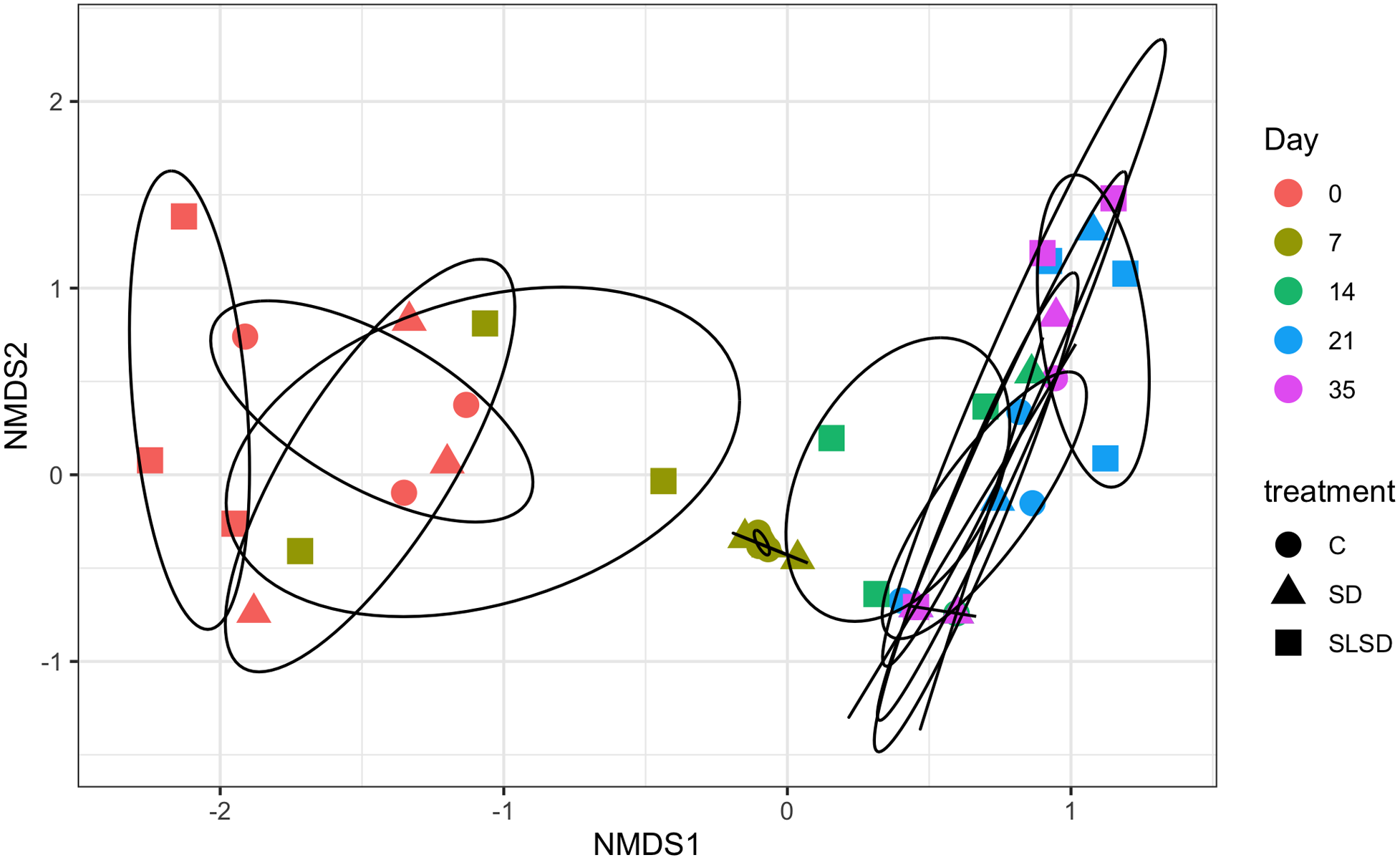
The dispersions within each treatment were similar (P = 0.57) satisfying the assumption to run PERMANOVA. PERMANOVA test results using Bray-Curtis and Jaccard distance matrix showed that there were no significant treatment main effects (P > 0.53). There was a treatment by day interaction (P = 0.001) demonstrating that the microbial community composition was affected significantly by treatment over storage time. Most variability was explained by the day between SLSD and C (P < 0.001), whereas other interactions were insignificant (P > 0.05). Very similar results were seen when using the Jaccard metric instead of Bray-Curtis. The differences between both the turkey breast samples within the group and across groups by plotting each day of sampling for all treatments, based on treatment type, were examined using NMDS of the Bray-Curtis dissimilarity index (Figure 2). The NMDS analysis indicated that the microbiota composition of samples in day 0 had a little diverse and spread-out pattern with a similar overall composition, whereas samples after day 14 clustered closely together. Analysis of turkey breast samples with different treatments showed a clear separation of SLSD from C and SD for especially day 0 and day 7, whereas C and SD were closely clustered. All treatments for days 14, 21, and 35 clustered at almost the same region.
Nonmetric multidimensional scaling (NMDS) ordination of β-diversity of microbiota community in turkey breast samples for 3 treatments from day 0 to day 35. Bray-Curtis distances were used between samples to generate NMDS to visualize microbiota dissimilarities. Each symbol in the figure represents the microbiota profile of a single sample. The same colored symbol represents groups of samples microbiota belongs to the same sampling day. C = control; SD = sodium diacetate; SLSD = sodium lactate + sodium diacetate. 0, 7, 14, 21, 35 = sampling days.
Composition
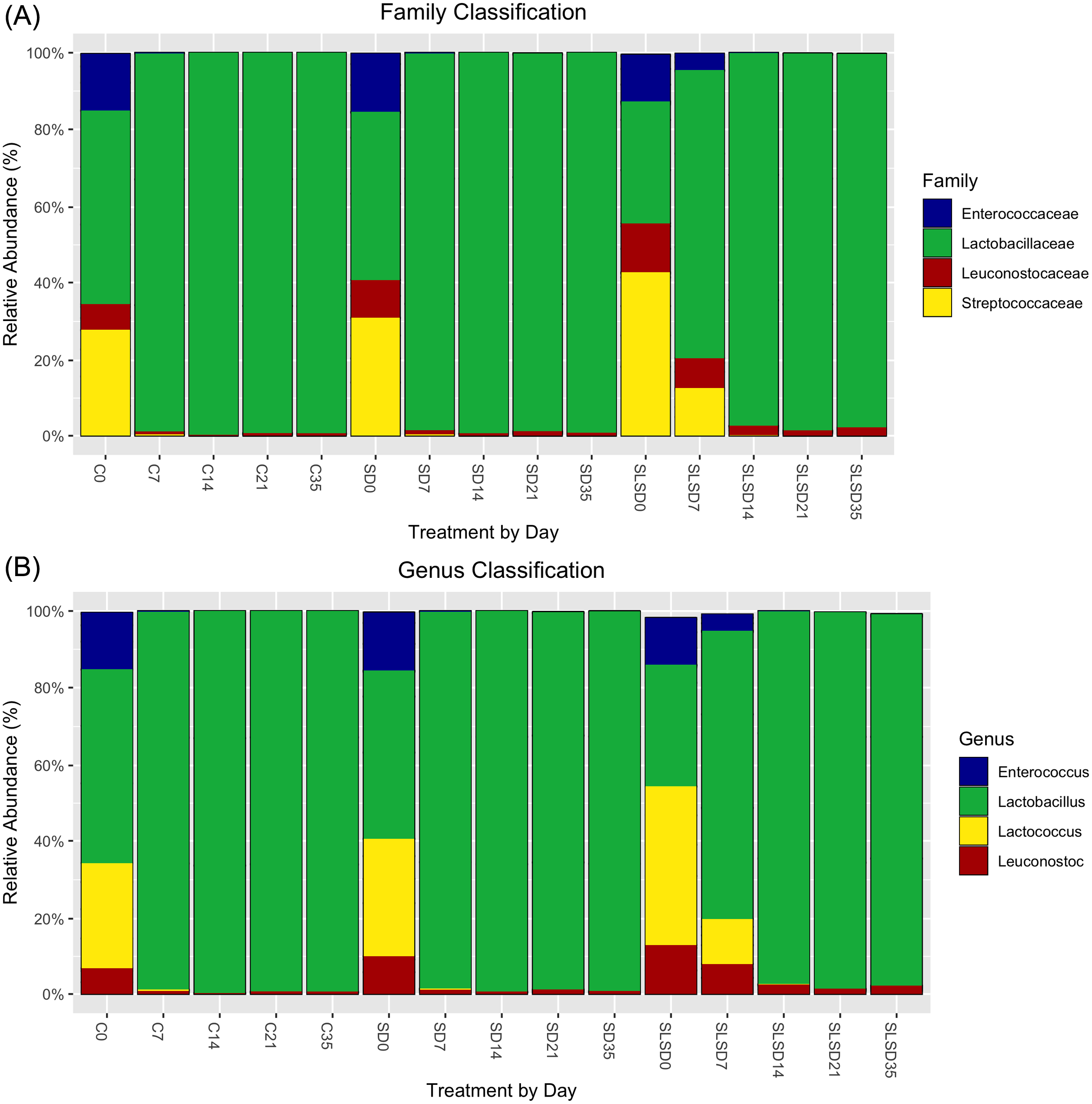
Taxonomic bar plots of family and genus-level classifications of OTUs (bacterial communities) based on different antimicrobial treatments and sampling day are demonstrated in Figure 3. Although the products were inoculated with equal concentrations (validated with the plate count method) of each strain of LAB, the relative abundances of each at day 0 were not found to be equal. From the family classification, all treatments were characterized by a relative increase in Lactobacillaceae from day 7, whereas Streptococcaceae and Enterococcaceae disappeared immediately after day 7. Leuconostocaceae population was significantly diminished through the storage time but remained almost the same after day 7. From genus classification, the most obvious change was a dramatic increase in Lactobacillus and a drastic decrease in all other genera for all treatments just after day 0. This decrease was a little slower for samples with SLSD compared with C and SD, which allowed SLSD samples to remain with a diverse community structure until day 14, but overall Lactobacillus (L. sakei) occupied the majority through 35 d of storage. Taxonomic analysis indicated that SD and C had similar microbial community structure changes at all sampling points.
Microbial structures of turkey breast samples. (A) Family-level classification. (B) Genus-level classification. Relative abundance of the inoculated 5 species used: Lactobacillus sakei, Lactococcus lactis, Enterococcus faecium, and 2 different Leuconostoc mesenteroides strains. C = control; SD = sodium diacetate; SLSD = sodium lactate + sodium diacetate. 0, 7, 14, 21, 35 = sampling days.
Discussion
At the beginning of the shelf life, there was a mixture of 5 added strains with equal concentrations of L. sakei, L. lactis, E. faecium, and 2 different L. mesenteroides strains (bacon and ham isolates) in the microbial community composition of turkey breast. Differences in relative abundances were observed at day 0, which might have occurred in the time interval from inoculation to sampling time because of the differences between adaptation capabilities of bacteria to the new environment. The mixture of the strains was still in a phase of rapid cell division when they were inoculated to turkey breast. Therefore, unequal distribution of bacteria might have occurred in a short time after inoculation although plate counts validated that similar and sufficient bacterial populations were added before inoculation. However, the ratios of LAB species abundances across all treatments were similar after day 14. If there is any significant antimicrobial effect of the organic acids, microbial growth and community composition would be expected to be different between control and the days following treatments. Overall, the changes in the microbial composition were similar for SD, SLSD, and control at the end of the 35 d. Only L. sakei and L. mesenteroides remained in the microbial community, whereas E. faecium and L. lactis totally disappeared after day 14. Possible high adaptation similarity of chosen bacteria to SD and SLSD or similar and weak mode of action of SD and SLSD might cause very similar community diversity and abundance as in control. Current study findings overlapped with the results of previous studies. Kalschne et al. (2015) reported that the predominant species in the vacuum-packaged RTE meat product were mainly Lactobacillus sp. followed by Leuconostoc sp. Nevertheless, it has been indicated that Enterococcus sp. was not present in the samples at the end of storage, although it was seen at the beginning of the shelf life in that study (Kalschne et al., 2015). It has been also reported that in a mixed culture dominance test, when L. mesenteriodies and L. sakei were present in equal initial numbers, L. sakei outcompeted L. mesenteroides at refrigeration temperatures (pH ≥ 6) (Zhang and Holley, 1999). Comi et al. (2016) also reported when L. mesenteroides were found with L. sakei or L. lactis bioprotective cultures, L. sakei or L. lactis grew faster than L. mesenteroides and the ratios were 1/1,000 (L. mesenteroides/L. sakei) or 1/100 (L. mesenteroides/L. lactis) at the end of shelf life in bacon, respectively. Therefore, the microbial richness of the meat and poultry products decreased during storage in both the current and cited studies. Only a few commonly reported species inside the community remained dominant to spoil the meat, which can be explained by competition for food and adaptation to the environment.
For treatments C, SD, and SLSD, L. sakei dominated the community by day 7, whereas relative abundances of L. lactis, E. faecium, and L. mesenteroides were dramatically decreased. SLSD slowed down the changes in microbial community structure until day 14. After day 14, SLSD had similar changes in relative abundances to C and SD. SLSD mixture might have delayed the lag phase of LAB for a short time until adaptation. If so, this effect probably came from the SL component itself because no effect was observed for SD. Similar to the current study, Drosinos et al. (2006) tested the antimicrobial effect of SL on spoilage LAB. They observed a limitation of bacterial growth when they used a concentration of 3% SL for 40 d at 4°C in cooked vacuum-packaged meat products. In addition, a significant change in the limitation effect of SL was observed in 4% and 5% SL concentration compared with 2% SL during 15 d in the broth system. However, bacteria behaved similarly to the control after day 15. Stekelenburg and Kant-Muermans (2001) reported similar effects with the application of SD (0.1% and 0.2%) and SL (2.5% and 3.3%) for spoilage LAB in cooked vacuum-packaged ham stored up to 40 d at 4°C. They reported an increase in lag time and shelf life with SL application, whereas no effect was observed with SD. Furthermore, shelf life doubled by 3.3% SL compared with 2.5% SL. The results in this study and literature offered the incorporation of SL in meat formulations had some antimicrobial effect on spoilage LAB microbiota and can extend shelf life, delaying the microbial growth. Higher lactate concentration may result in a more significant effect on growth dynamics on LAB. However, because of mimicking the industry practices in this study, similar amounts of antimicrobials preferred for L. monocytogenes in RTE meat products were used and limited our ability to more fully demonstrate this. Additionally, it was hard to draw any clear conclusion both on the interaction between other bacteria and the possible effect of SLSD because of the quick increase of L. sakei.
The findings of the current study were parallel with the results of the aerobic plate counts (APC) reported in our previous study at which SD and C displayed similar growth pattern in the microbiological challenge test, whereas SLSD had an up to 1-wk lag phase extension (results not shown here). The APC results were indicative; however, they did not tell how microbial community composition and abundance of LAB changed. Therefore, the behavior of microbes in RTE meats with different antimicrobials needed to be investigated at the family or genus level. The use of SD in the turkey breast formulation had similar results to the control and did not impact the microbial community composition through the shelf life. On the other hand, the presence of SLSD, compared with C and SD, influenced the relative abundances and slowed down the growth of LAB a little for the first week. In both studies, it might be concluded that SLSD had some negative impact on the growth of LAB by extending their lag phase for a short time. As known, lactate added to the meat system can slow cell growth by causing intracellular pH drop by increasing the dissociated lactic acid accumulation in the cytoplasm (Axe and Bailey, 1995; Ricke, 2003). It has been reported that lactate accumulation had an effect on the activity of lactate dehydrogenase (LDH) enzyme. Therefore, excess lactate in the cell needs to be extruded and this process requires energy. Cells canalize their energy to regulate these processes. Otherwise, it slows down LDH activity and disrupts pH homeostasis (Desguin et al., 2017). It is crucial for bacteria to survive rather than grow when the cytoplasmic pH is below neutral. In that case, bacteria spend their energy to regulate the cytoplasmic pH instead of proliferation. Bacteria cells in the log phase prefer to use their energy for pH regulation rather than growth in that process. However, cells in the adaptation period cannot properly handle the situation because they do not have enough energy, leading to the lag phase extension (de Wit and Rombouts, 1990).
Even though L. lactis had a similar predominance as L. sakei in SLSD compared with C and SD at day 0, later, L. sakei become better established in SLSD compared with the control, which confirmed that its dominance was not due to having a higher abundance at day 0 like in C and SD. It might also be concluded that not only dominance but also the existence of L. sakei might have a restricting factor on the growth of other bacteria. If so, L. mesenteroides was the most resistant LAB in this competition among others used in this study, surviving up to the end of the storage time. L. mesenteroides were also reported as dominant in some other studies and were responsible for spoilage with negative effects on the sausage products, such as off-color and off-flavor (Hultman et al., 2015; Comi et al., 2016; Weyker et al., 2016). However, in their microbial community composition, there was no L. sakei. Similarly, Leuconostoc sp. was reported as the most dominant species in minced meat supplemented with 2% SL under modified atmosphere when L. sakei was not present. Additionally, Lactococcus sp. were also present with Leuconostoc sp. in the minced meat at day 0. However, Lactococcus sp. had a very low relative abundance in a week and the results were similar to our current study (Stoops et al., 2015). No change was qualitatively observed in the color and odor of the turkey breast in this study even though a high growth of L. sakei was observed. Possible suppression of L. mesenteroides growth by L. sakei might prevent spoilage effect on the meat. Therefore, the results demonstrate the presence of L. sakei affected the microbiota.
Conclusions
The effect of organic acids as antimicrobials is widely known for pathogens but limited data are available for the effect of SL and SD on spoilage LAB in meat products in the literature. The results from this study can help to establish a better understanding of the interaction between bacterial growth dynamics and their behaviors under determined environmental conditions. However, comprehensive parallel studies are also needed to better unravel the effect of organic acids and the interactions between spoilage LAB at the species level in meat microbiota including their wide-scale concentrations. Knowledge from this and similar studies will lead to developing new approaches regarding the preservation of RTE meat products either in controlling meat spoilage or selecting bacteria to compete with spoilage or pathogen bacteria.
This study differs from other microbiota studies in the meat system. Instead of investigating natural background flora, the most common spoilage LAB community was defined (inoculated) beforehand. In this study, it was demonstrated the role of weak organic acid salts, SD and SLSD, on LAB responsible for meat spoilage. The effect of the SD and SLSD ranged from none to little in the spoilage community structure of RTE turkey breast by day 14. The spoilage patterns from day 14 to the end of day 35 were almost the same regardless of the applied organic acid. The overall microbial community was mainly dominated by L. sakei, and only a small decrease was observed in its growth pace until day 14 in treatment SLSD compared with others. This study demonstrates that the addition of SL into meat formulations slows down the changes in microbial community composition and abundance of its members.
Acknowledgements
This research was supported by the Food Research Institute at the University of Wisconsin-Madison and the General Directorate of Agricultural Research and Policies, Ministry of the Agriculture, Republic of Türkiye. The authors would like to thank them for their financial support. The authors declare no conflict of interest associated with this research.
Literature Cited
Anas, M., S. Ahmad, and A. Malik. 2019. Microbial escalation in meat and meat products and its consequences. In: A. Malik, Z. Erginkaya, and H. Erten, editors, Health and safety aspects of food processing technologies. Springer, Cham, Switzerland. p. 29–49. doi: https://doi.org/10.1007/978-3-030-24903-8_3
Axe, D. D., and J. E. Bailey. 1995. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8–19. doi: https://doi.org/10.1002/bit.260470103
Barakat, R. K., M. W. Griffiths, and L. J. Harris. 2000. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. Int. J. Food Microbiol. 62:83–94. doi: https://doi.org/10.1016/S0168-1605(00)00381-0
Berry, D. 2019. Meat processors reducing food waste by extending shelf life. Food Business News. https://www.foodbusinessnews.net/articles/13490-meat-processors-reducing-food-waste-by-extending-shelf-life. (Accessed 19 October 2020.)
Borch, E., M.-L. Kant-Muermans, and Y. Blixt. 1996. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 33:103–120. doi: https://doi.org/10.1016/0168-1605(96)01135-x
Cauchie, E., L. Delhalle, B. Taminiau, A. Tahiri, N. Korsak, S. Burteau, P. A. Fall, F. Farnir, G. Baré, and G. Daube. 2020. Assessment of spoilage bacterial communities in food wrap and modified atmospheres-packed minced pork meat samples by 16S rDNA metagenetic analysis. Front. Microbiol. 10. doi: https://doi.org/10.3389/fmicb.2019.03074
Chao, A. 1984. Nonparametric estimation of the classes in a population. Scand. J. Stat. 11:265–270.
Chávez-Martínez, A., M. Estrada Gandarilla, A. L. Rentería-Monterrubio, M. A. Gallegos Acevedo, and J. C. Rodríguez Figueroa. 2016. Prevalence of lactic acid bacteria in sliced cooked ham as an indicator of its shelf life. Vitae. 23:38–46. doi: https://doi.org/10.17533/udea.vitae.v23n3a02
Chenoll, E., M. C. Macián, P. Elizaquível, and R. Aznar. 2007. Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods. J. Appl. Microbiol. 102:498–508. doi: https://doi.org/10.1111/j.1365-2672.2006.03081.x
Comi, G., D. Andyanto, M. Manzano, and L. Iacumin. 2016. Lactococcus lactis and Lactobacillus sakei as bio-protective culture to eliminate Leuconostoc mesenteroides spoilage and improve the shelf life and sensorial characteristics of commercial cooked bacon. Food Microbiol. 58:16–22. doi: https://doi.org/10.1016/j.fm.2016.03.001
de Wit, J. C., and F. M. Rombouts. 1990. Antimicrobial activity of sodium lactate. Food Microbiol. 7:113–120. doi: https://doi.org/10.1016/0740-0020(90)90017-C
Dempster, J. F., S. N. Reid, and O. Cody. 1973. Sources of contamination of cooked, ready-to-eat cured and uncured meats. J. Hyg.-Cambridge. 71:815–823. doi: https://doi.org/10.1017/S002217240002307X
Drosinos, E. H., M. Mataragas, A. Kampani, D. Kritikos, and I. Metaxopoulos. 2006. Inhibitory effect of organic acid salts on spoilage flora in culture medium and cured cooked meat products under commercial manufacturing conditions. Meat Sci. 73:75–81. doi: https://doi.org/10.1016/j.meatsci.2005.11.003
Dykes, G. A., T. J. Britz, and A. von Holy. 1994. Numerical taxonomy and identification of lactic acid bacteria from spoiled, vacuum-packaged vienna sausages. J. Appl. Bacteriol. 76:246–252. doi: https://doi.org/10.1111/j.1365-2672.1994.tb01623.x
Edgar, R. C., B. J. Haas, J. C. Clemente, C. Quince, and R. Knight. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 27:2194–2200. doi: https://doi.org/10.1093/bioinformatics/btr381
Ercolini, D. 2013. High-throughput sequencing and metagenomics: moving forward in the culture-independent analysis of food microbial ecology. Appl. Environ. Microb. 79:3148–3155. doi: https://doi.org/10.1128/AEM.00256-13
Falowo, A. B., P. O. Fayemi, and V. Muchenje. 2014. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: a review. Food Res. Int. 64:171–181. doi: https://doi.org/10.1016/j.foodres.2014.06.022
Glass, K. A., D. A. Granberg, A. L. Smith, A. M. McNamara, M. Hardin, J. Mattias, K. Ladwig, and E. A. Johnson. 2002. Inhibition of Listeria monocytogenes by sodium diacetate and sodium lactate on wieners and cooked bratwurst. J. Food Protect. 65:116–123. doi: https://doi.org/10.4315/0362-028X-65.1.116
Good, I. J. 1953. The population frequencies of species and the estimation of population parameters. Biometrika. 40:237–264. doi: https://doi.org/10.2307/2333344
Hamasaki, Y., M. Ayaki, H. Fuchu, M. Sugiyama, and H. Morita. 2003. Behavior of psychrotrophic lactic acid bacteria isolated from spoiling cooked meat products. Appl. Environ. Microb. 69:3668–3671. doi: https://doi.org/10.1128/AEM.69.6.3668-3671.2003
Hultman, J., R. Rahkila, J. Ali, J. Rousu, and K. J. Björkroth. 2015. Meat processing plant microbiome and contamination patterns of cold-tolerant bacteria causing food safety and spoilage risks in the manufacture of vacuum-packaged cooked sausages. Appl. Environ. Microb. 81:7088–7097. doi: https://doi.org/10.1128/AEM.02228-15
Iulietto, M. F., P. Sechi, E. Borgogni, and B. T. Cenci-Goga. 2015. Meat spoilage: a critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 14:4011. doi: https://doi.org/10.4081/ijas.2015.4011
Kalschne, D. L., R. Womer, A. Mattana, C. M. P. Sarmento, L. M. Colla, and E. Colla. 2015. Characterization of the spoilage lactic acid bacteria in “sliced vacuum-packed cooked ham.” Braz. J. Microbiol. 46:173–181. doi: https://doi.org/10.1590/S1517-838246120130019
Karwowska, M., S. Łaba, and K. Szczepański. 2021. Food loss and waste in meat sector—Why the consumption stage generates the most losses? Sustainability-Basel. 13:6227. doi: https://doi.org/10.3390/su13116227
Korkeala, H. J., and K. J. Björkroth. 1997. Microbiological spoilage and contamination of vacuum-packaged cooked sausages. J. Food Protect. 60:724–731. doi: https://doi.org/10.4315/0362-028X-60.6.724
Kozich, J. J., S. L. Westcott, N. T. Baxter, S. K. Highlander, and P. D. Schloss. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microb. 79:5112–5120. doi: https://doi.org/10.1128/AEM.01043-13
Maks, N., L. Zhu, V. K. Juneja, and S. Ravishankar. 2010. Sodium lactate, sodium diacetate and pediocin: effects and interactions on the thermal inactivation of Listeria monocytogenes on bologna. Food Microbiol. 27:64–69. doi: https://doi.org/10.1016/j.fm.2009.08.004
Mbandi, E., and L. A. Shelef. 2002. Enhanced antimicrobial effects of combination of lactate and diacetate on Listeria monocytogenes and Salmonella spp. in beef bologna. Int. J. Food Microbiol. 76:191–198. doi: https://doi.org/10.1016/S0168-1605(02)00026-0
McMurdie, P. J., and S. Holmes. 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 8:e61217. doi: https://doi.org/10.1371/journal.pone.0061217
Odeyemi, O. A., O. O. Alegbeleye, M. Strateva, and D. Stratev. 2020. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. F. 19:311–331. doi: https://doi.org/10.1111/1541-4337.12526
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. Mcglinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens, E. Szoecs, and H. W. Maintainer. 2019. vegan: Community Ecology Package. R package version 2.5-5. https://CRAN.R-project.org/package=vegan.
Pellissery, A. J., P. G. Vinayamohan, M. A. R. Amalaradjou, and K. Venkitanarayanan. 2020. Chapter 17 - Spoilage bacteria and meat quality. In: A. K. Biswas and P. K. Mandal, editors, Meat Quality Analysis. Academic Press, London. p. 307–334. doi: https://doi.org/10.1016/B978-0-12-819233-7.00017-3
Pothakos, V., G. Stellato, D. Ercolini, and F. Devlieghere. 2015. Processing environment and ingredients are both sources of Leuconostoc gelidum, which emerges as a major spoiler in ready-to-eat meals. Appl. Environ. Microb. 81:3529–3541. doi: https://doi.org/10.1128/AEM.03941-14
Pruesse, E., C. Quast, K. Knittel, B. M. Fuchs, W. Ludwig, J. Peplies, and F. O. Glöckner. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196. doi: https://doi.org/10.1093/nar/gkm864
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ricke, S. C. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Sci. 82:632–639. doi: https://doi.org/10.1093/ps/82.4.632
Rouger, A., O. Tresse, and M. Zagorec. 2017. Bacterial contaminants of poultry meat: sources, species, and dynamics. Microorganisms. 5:50. doi: https://doi.org/10.3390/microorganisms5030050
Samelis, J., A. Kakouri, K. G. Georgiadou, and J. Metaxopoulos. 1998. Evaluation of the extent and type of bacterial contamination at different stages of processing of cooked ham. J. Appl. Microbiol. 84:649–660. doi: https://doi.org/10.1046/j.1365-2672.1998.00392.x
Samelis, J., A. Kakouri, and J. Rementzis. 2000a. Selective effect of the product type and the packaging conditions on the species of lactic acid bacteria dominating the spoilage microbial association of cooked meats at 4°C. Food Microbiol. 17:329–340. doi: https://doi.org/10.1006/fmic.1999.0316
Samelis, J., A. Kakouri, and J. Rementzis. 2000b. The spoilage microflora of cured, cooked turkey breasts prepared commercially with or without smoking. Int. J. Food Microbiol. 56:133–143. doi: https://doi.org/10.1016/S0168-1605(99)00190-7
Samelis, J., G. K. Bedie, J. N. Sofos, K. E. Belk, J. A. Scanga, and G. C. Smith. 2005. Combinations of nisin with organic acids or salts to control Listeria monocytogenes on sliced pork bologna stored at 4°C in vacuum packages. LWT-Food Sci. Technol. 38:21–28. doi: https://doi.org/https://doi.org/10.1016/j.lwt.2004.04.012
Sarmento, C. M. P., E. Colla, C. Canan, F. Dalcanton, and G. M. F. de Aragão. 2015. Food additives reduce lactic acid bacterial growth in culture medium and in meat products, increasing product shelf life. Semina: Ciências Agrárias. 36:3681–3697. doi: https://doi.org/10.5433/1679-0359.2015v36n6p3681
Schloss, P. D., S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn, and C. F. Weber. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 75:7537–7541. doi: https://doi.org/10.1128/AEM.01541-09
Shannon, C. E. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27:379–423. doi: https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Sofos, J. N. 2014. Chapter 6 - Meat and meat products. In: Y. Motarjemi and H. Lelieveld, editors, Food safety management: a practical guide for the food industry, Academic Press, San Diego, CA. p. 119–162. doi: https://doi.org/10.1016/B978-0-12-381504-0.00006-8
Stekelenburg, F. K., and M. L. T. Kant-Muermans. 2001. Effects of sodium lactate and other additives in a cooked ham product on sensory quality and development of a strain of Lactobacillus curvatus and Listeria monocytogenes. Int. J. Food Microbiol. 66:197–203. doi: https://doi.org/10.1016/S0168-1605(00)00521-3
Stoops, J., S. Ruyters, P. Busschaert, R. Spaepen, C. Verreth, J. Claes, B. Lievens, and L. Van Campenhout. 2015. Bacterial community dynamics during cold storage of minced meat packaged under modified atmosphere and supplemented with different preservatives. Food Microbiol. 48:192–199. doi: https://doi.org/10.1016/j.fm.2014.12.012
Stevenson, D. M., and P. J. Weimer. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biot. 75:165–174. doi: https://doi.org/10.1007/s00253-006-0802-y
Stopforth, J. D., D. Visser, R. Zumbrink, L. Van Dijk, and E. W. Bontenbal. 2010. Control of Listeria monocytogenes on cooked cured ham by formulation with a lactate-diacetate blend and surface treatment with lauric arginate. J. Food Protect. 73:552–555. doi: https://doi.org/10.4315/0362-028X-73.3.552
USDA. 2016. Sanitation concerns in RTE processing environments. https://www.fsis.usda.gov/sites/default/files/media_file/2021-11/28_IM_RTE-Sanitation-11292016.pdf. (Accessed 2 December 2020.)
Weyker, R. E., K. A. Glass, A. L. Milkowski, D. L. Seman, and J. J. Sindelar. 2016. Controlling Listeria monocytogenes and Leuconostoc mesenteroides in uncured deli-style turkey breast using a clean label antimicrobial. J. Food Sci. 81:M672–M683. doi: https://doi.org/10.1111/1750-3841.13232
Wickham, H. 2009. ggplot2: elegant graphics for data analysis. Springer-Verlag New York.
Yang R., and B. Ray. 1994. Prevalence and biological control of bacteriocin-producing psychrotrophic leuconostocs associated with spoilage of vacuum-packaged processed meats. J. Food Protect. 57:209–217. doi: https://doi.org/10.4315/0362-028x-57.3.209
Zhang, G., and R. A. Holley. 1999. Development and PFGE monitoring of dominance among spoilage lactic acid bacteria from cured meats. Food Microbiol. 16:633–644. doi: https://doi.org/10.1006/fmic.1999.0281
Zhao, F., G. Zhou, K. Ye, S. Wang, X. Xu, and C. Li. 2015. Microbial changes in vacuum-packed chilled pork during storage. Meat Sci. 100:145–149. doi: https://doi.org/10.1016/j.meatsci.2014.10.004