Introduction
Meat discoloration during retail display is a process characterized by changes in myoglobin (Mb), the main pigment in meat. Normally, Mb on the meat surface is bound to oxygen (oxymyoglobin [OMb]) and appears red in color, with the color intensity depending on species (King et al., 2023). However, Mb can be oxidized to form metmyoglobin (MMb), resulting in development of a brown or gray color. Consumers discriminate against meat that appears brown or gray in color, resulting in profit losses and potential discarding of product (Font-i-Furnols and Guerrero, 2014). When evaluating color stability of meat, MMb content is often used as an indicator of discoloration extent (Mancini and Hunt, 2005). Discoloration in pork is influenced by initial color traits, including lightness, redness, and hue angle (Zhu and Brewer, 1998; King et al., 2011a).
In beef, discoloration is muscle dependent. Multiple studies have reported that the psoas major (PM) and triceps brachii (TB) were more color labile than the longissimus lumborum (LL), and that color labile muscles had greater initial redness and Mb content and consumed more oxygen during display (McKenna et al., 2005; Jeong et al., 2009; King et al., 2011b; Mancini et al., 2018). Muscle color stability differences can be partially attributed to muscle fiber differences, as color labile muscles possess predominantly red, oxidative fibers whereas color stable muscles primarily possess white, glycolytic fibers (Faustman et al., 2010). Despite differences between muscles, King et al. (2011b) observed relationships between the LL and other muscles for color stability, indicating that factors affecting the entire carcass like genetics, diet, or processing conditions may exert influence over color stability.
Studies regarding the color stability of different pork muscles are much more limited compared to beef. However, previous studies have reported color and muscle fiber type differences between pork muscles. Cheng et al. (2021) observed that pork PM was darker, redder, and had a greater proportion of oxidative fibers compared to pork longissimus dorsi (LD). Souza et al. (2011) did not evaluate muscle fiber type, but observed that the TB was darker and redder than the LD. If observations from beef regarding differences in color stability hold true, redder, more oxidative muscles like the TB and PM would be expected to be less color stable than the LD in pork. Furthermore, if factors controlling color stability in pork muscles are similar to beef, then color stability of the LD in pork may be indicative of color stability for other muscles as well. Therefore, the objectives of this study were to evaluate differences in color stability among the TB, PM, and LD muscles and relate those differences to biochemical traits. Using this information, a secondary objective was to determine whether color changes in the LD were related to color changes in other muscle types. It was hypothesized that, similar to observations in beef, TB and PM would discolor more quickly than the LD and that changes in LD color during display would be related to color changes in the TB and PM.
Materials and Methods
Pigs used in this study were the offspring of sire lines representing Pietrain ancestry (Choice Genetics USA, West Des Moines, IA). Boars were mated to Camborough sows (Pig Improvement Company, Hendersonville, TN), and parity of the females was balanced among sire lines. Pigs were housed in single-sex pens by sire line with 4 pigs housed per pen. A total of 80 pens were allocated for another experiment, and the second heaviest pig in 20 pens was selected for slaughter. All pigs received an industry-typical corn-soy finishing diet. All protocols were approved by the University of Illinois Institutional Animal Care and Use Committee (Protocol #20095).
Sample preparation
Pigs (n = 20) were transported to the University of Illinois Meat Science Laboratory and held in lairage for approximately 16 h with no feed and ad libitum access to water. Pigs were slaughtered under the supervision of the USDA Food and Safety Inspection Service. Pigs were immobilized by electrical stunning and terminated by exsanguination. Carcasses were allowed to chill for approximately 22 h at 4°C. Left carcass sides were fabricated using North American Meat Processors (NAMP; 2014) to yield the picnic shoulder (NAMP #405) and bone-in loin (NAMP #410). Picnic shoulders were further fabricated to yield the TB, and loins were fabricated to yield the LD and PM. The TB and PM were individually vacuum packaged and aged for 21 d at 4°C. The LD was cut into 2.54 cm chops using a push-feed slicer (Treif model 700 F, Oberlahr, Germany). The 3 chops cut immediately posterior to the 10th rib were vacuum packaged together and stored in the same manner as TB and PM chops. At the end of the aging period, TB and PM muscles were cut by hand to yield 3 chops measuring approximately 2.54 cm and 5.08 cm in thickness, respectively. Ultimate pH of the first chop for each muscle was measured using a Hanna pH meter fitted with a glass-tipped electrode (model HI98163, Hanna Instruments, Woonsocket, RI) calibrated using pH 4 and pH 7 buffer at 4°C.
Retail display
All 3 chops from a pig representing a single muscle were placed on a 27.3 × 14.9 cm polystyrene tray (Dyne-a-pak, Laval, Quebec, Canada) with a soaker pad and overwrapped with polyvinyl chloride film (O2 transmission = 23,250 mL · m2 · d-1, 72 gauge; Resinite Packaging Films, Borden, Inc., North 70 Andover, MA). The cut surface of chops was oriented to be visible through the film. Within a package, chops were randomly allotted to 1, 3, or 5 d of display in the package, and they were removed from display at that point for biochemical analyses. Packages were arranged on 4 wire mesh shelves in a standing display cooler and displayed at 4°C. Lighting was provided by 122 cm fluorescent bulbs (32 W, Octron XP, 6500 K, Osram Sylvania, Wilmington, MA) positioned at the front of the display case. Packages were rotated each day to minimize location effects on discoloration. Samples were allowed to oxygenate for at least 2 h prior to color evaluation on the first day of display. On each day of display, subjective and instrumental discoloration were evaluated on all chops in a package. Discoloration of chops within a single package were then averaged for statistical analyses.
Visual discoloration was evaluated through the film by a panel of 8 trained technicians using a 10-cm line scale anchored at 0%, 50%, and 100% discoloration. Instrumental surface color including CIE lightness (L*), redness (a*), and yellowness (b*; CIE, 1976) and reflectance data (400–700 nm) were evaluated using a HunterLab MiniScan EZ spectrophotometer (HunterLab, Reston, VA) as described by King et al. (2023). The spectrophotometer was equipped with a D65 illuminant and 31.8 mm aperture and was calibrated with black and white tiles specific to the machine. Film was removed from packages prior to instrumental evaluation. Instrumental L*, a*, and b* data were used to calculate chroma (measure of saturation: ) and hue angle (measure of color hue: . Reflectance data were used to calculate the ratio of 630/580 nm (an indicator of surface brownness) and Mb forms using the attenuance method as described by Krzywicki (1979). Reflectances at 474, 525, 572, and 700 nm were converted to attenuance values (A) using the equation A = log(1/R), where R = reflectance. Myoglobin forms were calculated using the following equations:
Biochemical analyses
On their respective day of analysis (day 1, 3, or 5), chops for each muscle were removed from display and trimmed of all external fat. Chops from the LD and TB were cut in half perpendicular to the chop’s face surface, while PM chops were cut in half parallel to the cut surface. This was done to ensure muscle fibers ran in the same direction for all muscle samples. One of these halves was frozen at −80° C to be used for Mb quantification (day 1 only) and lipid oxidation, while the remaining half was used to determine oxygen consumption (OC) and MMb reducing capacity (MRA). Sample portions reserved for OC and MRA were cut in half parallel to the muscle fiber direction, and halves were randomly allotted to each analysis.
Oxygen consumption
Oxygen consumption was determined as described by King et al. (2023) with minor modifications. The freshly cut surface of the OC sample was covered with PVC film and allowed to oxygenate at 4°C for 2 h. After oxygenation, samples were vacuum packaged and immediately scanned through the oxygen impermeable film with the same Hunter spectrophotometer used for instrumental color evaluations. After packaging, samples were allowed to incubate at 20°C for 1 h, then scanned a second time with the Hunter. The proportion of OMb on oxygenated and deoxygenated samples was determined using the Krzywicki (1979) method as previously outlined. The percentage oxygen consumed was calculated using the initial and final OMb content of each sample as outlined by King et al. (2023):
Metmyoglobin reducing activity
Metmyoglobin reducing activity was determined as described by King et al. (2023) with minor modifications. The freshly cut surface of the sample designated for MRA was submerged in a solution of 0.3% sodium nitrite solution for 30 min at approximately 20°C. The sample was then removed from the solution, blotted dry, and vacuum packaged. Samples were immediately scanned with the Hunter spectrophotometer through the film after sealing, then incubated at 20°C for 3 h. Samples were scanned through the film a second time after the incubation period was complete. Proportions of MMb during initial and final measurements were determined using the Krzywicki (1979) method. The percentage MRA was calculated using the initial and final MMb content of each sample as outlined by King et al. (2023):
Myoglobin content
Myoglobin content was determined on samples removed on day 1 of display according to the method outlined by Faustman and Phillips (2001). Samples were diced and pulverized into a fine powder using liquid nitrogen. Duplicate 5 g powdered samples were homogenized in 45 mL ice cold 40 mM potassium phosphate buffer (pH = 6.8) and filtered through Whatman no. 1 filter paper. After filtering, 150 μL of the filtrate was pipetted into a 96-well plate with potassium phosphate buffer as a blank. The absorbance of the filtrate was measured at 525 nm using a plate reader (Synergy HT Multi-Model Microplate Reader, Bio-Tek, Winooski, VT). Myoglobin content was determined using the following equation:
where A525 = absorbance at 525 nm, 7.6 mM−1 cm−1 =millimolar extinction coefficient of Mb at 525 nm; 1 cm =path length of cuvette; 17,000 Da = average molecular mass of Mb, and the dilution factor is 10.Lipid oxidation
Samples were prepared for lipid oxidation analysis in the same manner as for Mb content. Lipid oxidation was evaluated using the thiobarbituric acid reactive substances (TBARS) assay as outlined by Leick et al. (2010) with modifications. Duplicate 5 g powdered samples were homogenized with 1 mL of 0.2 mg/mL butylated hydroxytoluene and 45.5 mL of 10% trichloroacetic acid in 0.2 M phosphoric acid, then filtered through Whatman no. 1 filter paper. Two 5-mL aliquots of the filtrate were transferred into 15-mL conical tubes. Five mL of 0.02 M thiobarbituric acid was added to one tube while 5 mL of deionized water was added to the second to serve as a blank. A standard curve was also made to represent 0, 1.25, 2.5, 5, and 7.5 mg malondialdehyde (MDA)/mL using 25 μM 1,1,3,3-tetraethoxypropane. Samples were then allowed to incubate in the dark for approximately 17 h at 23°C. Afterward, 150 μL of the sample and blank were pipetted into 96-well flat-bottomed plates. Sample absorbance was read at 530 nm using a plate reader and compared to the standard curve to determine mg MDA content per kg meat.
Statistical analyses
Data were analyzed using the MIXED procedure of SAS (v. 9.4; SAS Institute Inc., Cary, NC). Retail display data were analyzed as a repeated measure in time. The model included muscle and display day as fixed effects, as well as interactions between the two. A compound symmetry covariance structure was chosen using Akaike’s information criterion to minimize variance. Overall changes in color and biochemical measurements during the 5-d display period were calculated and analyzed as a 1-way ANOVA with muscle serving as a fixed effect and carcass as a random effect. Least-squares means were separated using the PDIFF option of the MIXED procedure. Ultimate pH and Mb content were analyzed as a 1-way ANOVA with muscle as a fixed effect and carcass as a random effect. For each day of display, the percentage of samples for each muscle type considered “acceptable” (<20% visual discoloration score) was calculated. This percentage was chosen because 20% is the amount of MMb commonly referenced, in beef, as the threshold at which consumers begin to discriminate against discoloration (Renerre and Labas, 1987; Gill and Jones, 1994). Differences in percentages of acceptable samples between muscles were determined using the FREQ procedure of SAS. Main effects and interactions were considered different at P < 0.05 and trending at 0.05 < P < 0.10.
Pearson correlation coefficients were calculated using the CORR procedure of SAS to determine relationships between changes in LD measurements over time (day 5 − day 1) and changes in PM or TB measurements over time. Correlations were considered statistically significant at P < 0.05 and trending at 0.05 < P < 0.10. Significant correlations were considered weak at r < |0.35|, moderate at |0.36| ≥ r < |0.67|, and strong at r ≥ |0.68|, similar to Taylor (1990).
Results
On day 1 of display, muscle affected (P ≤ 0.05) all starting color and biochemical traits except for visual discoloration and lipid oxidation (P ≥ 0.10; Table 1). Chops from the LD were 11.7 to 14.6 L* units lighter, 5.6 to 6.9 a* units less red, and 2.4 to 4.4 chroma units less saturated; had hue angles 11.8 to 13.2 units greater; and had 630/580 nm ratios approximately 1.5 units greater than TB and PM chops on day 1 of retail display (P < 0.01). Furthermore, on day 1, LD chops had on average lesser MMb concentrations, greater OMb concentrations, less OC, greater MRA, and less total Mb and had lower pH values than TB and PM chops (P < 0.01). Chops from the PM were 2.3 units more yellow than TB chops, with LD chops intermediate (P < 0.01). Deoxymyoglobin of TB chops was greater compared to LD and PM chops (P < 0.01); however, DMb concentrations did not differ between the LD and PM (P = 0.16). Chops from the PM were 2.9 L* units lighter, 1.3 a* units redder, and 2.5 chroma units more saturated and had 1.4-unit-greater hue angles than chops from the TB. Chops for the PM also had lesser MMb concentrations, reduced MRA, and less total Mb than chops from the TB (P ≤ 0.04). Alternatively, TB and PM chops did not differ in day 1 630/580 nm ratio, OC, or pH (P ≥ 0.15).
Effect of muscle on initial (day 1) color and biochemical traits of fresh pork during display1,2
| Item | LD | TB | PM | SEM | P Value |
|---|---|---|---|---|---|
| Samples, n | 20 | 20 | 20 | ||
| Lightness, L* | 59.10a | 44.51c | 47.41b | 0.70 | <0.01 |
| Redness, a* | 11.52c | 17.11b | 18.43a | 0.25 | <0.01 |
| Yellowness, b* | 18.57b | 17.17c | 19.43a | 0.31 | <0.01 |
| Chroma3 | 21.87c | 24.26b | 26.79a | 0.35 | <0.01 |
| Hue Angle4 | 58.24a | 45.07c | 46.44b | 0.44 | <0.01 |
| 630/580 nm | 2.83b | 4.31a | 4.36a | 0.06 | <0.01 |
| MMb, %5 | 11.9c | 19.3a | 17.0b | 0.31 | <0.01 |
| DMb, %6 | 3.8b | 13.6a | 6.3b | 1.90 | <0.01 |
| OMb, %7 | 84.3a | 67.1c | 76.7b | 1.67 | <0.01 |
| Visual Discoloration, % | 1.8 | 2.1 | 3.3 | 0.57 | 0.10 |
| Oxygen Consumption, % | 59.4b | 99.6a | 96.0a | 3.98 | <0.01 |
| Metmyoglobin Reducing Activity, % | 49.0a | 40.6b | 35.6c | 2.34 | <0.01 |
| mg MDA/kg Meat | 0.02 | 0.01 | 0.01 | 0.01 | 0.67 |
| mg Myoglobin/g Meat | 0.7c | 2.1a | 1.2b | 0.12 | <0.01 |
| pH | 5.61b | 5.90a | 5.84a | 0.04 | <0.01 |
Treatments within a row lacking common superscripts are different (P < 0.05).
Means for the longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM).
Samples were allowed to oxygenate for at least 2 h prior to color evaluation on day 1.
Chroma is a measure of color saturation, calculated by C = (a*2 + b*2)1/2.
Hue angle is a measure of primary color hue of a sample, calculated by Hab = arctangent (b*/a*).
Calculated as follows: .
Calculated as follows: .
Calculated as follows: %OMb = 100 − %MMb − %DMb.
DMb = deoxymyoglobin; MDA = malondialdehyde; MMb = metmyoglobin; OMb = oxymyoglobin.
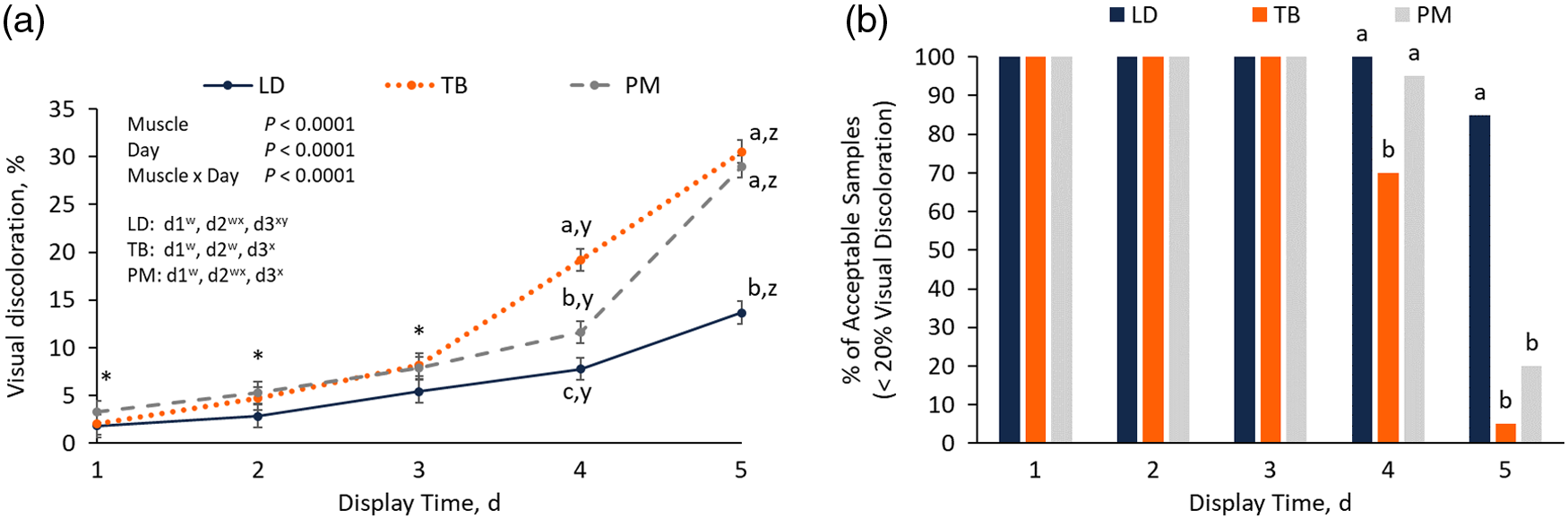
A muscle × day interaction was also observed for visual discoloration (P < 0.01; Figure 1A). No differences in visual discoloration were observed among muscles on the first 3 d of display (P ≥ 0.30). From day 3 to day 5, visual discoloration of LD chops increased by 8.2 units whereas discoloration of PM and TB chops increased by 21.1 and 22.3 units, respectively (P < 0.01). Both TB and PM chops had greater final (day 5) discoloration than LD chops (P < 0.01) as well as greater overall changes (day 5 − day 1) in visual discoloration compared to LD (P < 0.01), but they did not differ from each other (P = 0.29; Table 1). In the current study, individual samples were considered “unacceptable” for display once average visual discoloration scores reached at least 20% (Figure 1B). On day 1, day 2, and day 3, 100% of samples for each muscle were considered acceptable. However, on day 4, TB chops had the smallest proportion of acceptable samples (70%), while 100% of LD samples and 95% of PM samples were considered acceptable (P < 0.01). On day 5, LD had the greatest proportion of acceptable samples (85%), whereas only 5% of TB samples and 20% of PM samples were still considered acceptable (P < 0.01). Overall, TB and PM chops were less color stable than LD chops, especially from day 3 to day 5 of display.
Effect of muscle on visual discoloration (A) of pork longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) chops over 5 d of retail display, and percentage of samples considered “acceptable” (<20% visual discoloration) on a given display day (B). Within a day of display, different letters (a–c) indicate a significant effect of muscle (P < 0.05). Within a muscle, different letters (w–z) indicate a significant effect of day (P < 0.05).
Lightness decreased during display for all muscles evaluated (P < 0.01), with no muscle × day interaction for instrumental lightness (P = 0.69; Figure 2A). On all days of display, LD chops were at least 11.7 L* units lighter than PM and TD chops (P < 0.01). Muscle × day interactions were observed for redness (Figure 2B) and yellowness (Figure 2C) during display (P < 0.01). On day 1 of display, PM chops were 1.3 a* units redder than TB chops and 6.9 a* units redder than LD chops (P < 0.01). However, on day 5 of display, PM and TB chops were only 2.3 and 3.4 a* units redder than LD chops, respectively (P < 0.01). Chops from the TB and PM experienced greater declines in redness (Δ a* = 5.4 and 5.6, respectively) over the display period when compared to LD chops (Δ a* = 2.1; P < 0.01), but they did not differ from each other (P = 0.64). There was also a greater decline in yellowness of chops from the TB and PM (Δ b* = 3.0 and 3.3, respectively) compared to LD chops (Δ b* = 1.1; P < 0.01).
Effect of muscle on instrumental lightness (A), redness (B), and yellowness (C) of pork longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) chops over 5 d of retail display. Within a day of display, different letters (a–c) indicate a significant effect of muscle (P < 0.05). Within a muscle, different letters (v–z) indicate a significant effect of day (P < 0.05).
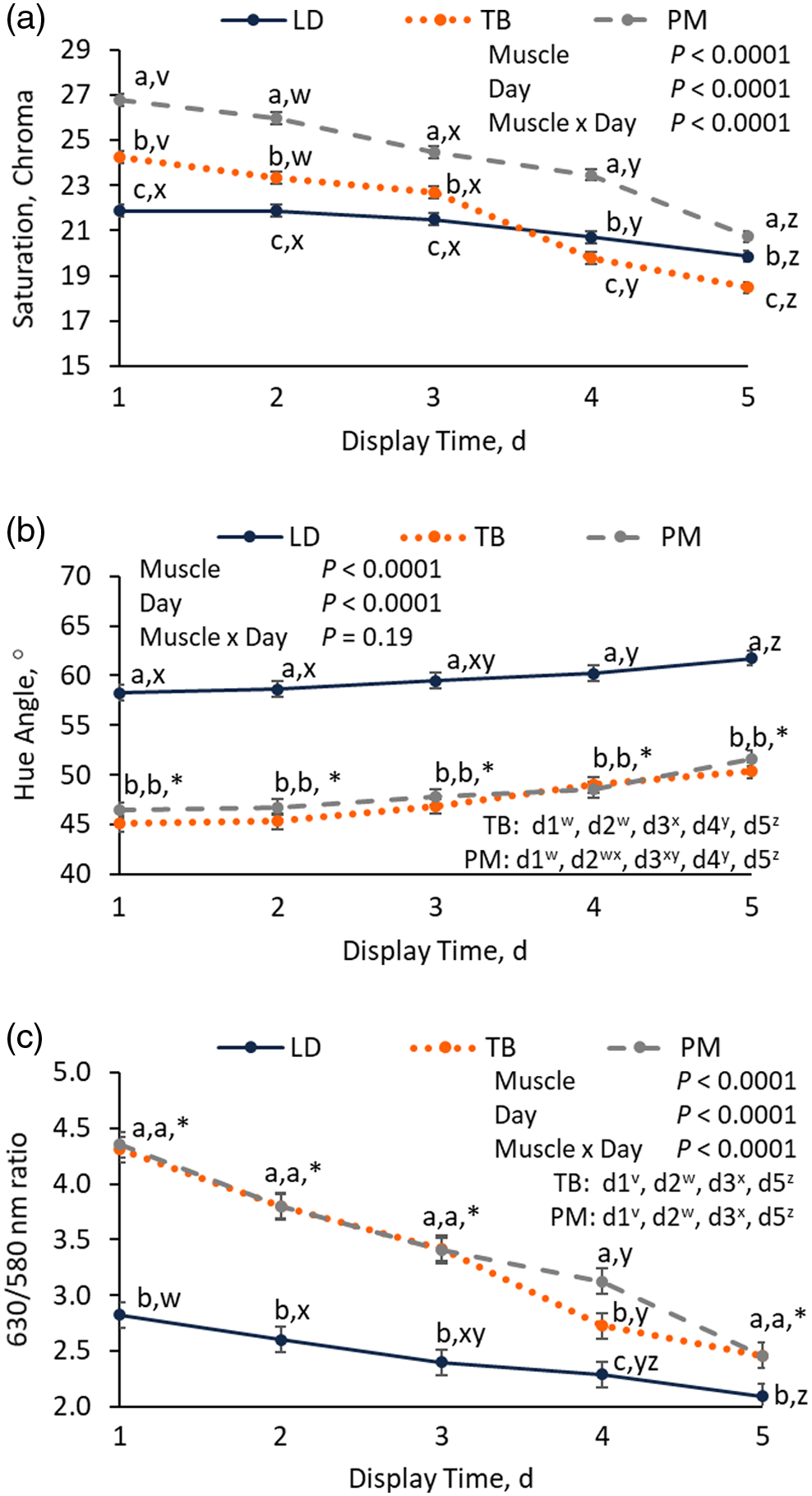
A muscle × day interaction (P < 0.0001) was observed for chroma (Figure 3A). Chops from the TB had intermediate chroma values to LD and PM chops on day 1 to day 3 of display (P < 0.01) but had the lowest chroma values by days 4 and 5 (P < 0.01). Chops from the TB and PM had greater decreases in chroma over the display period (Δ chroma = 5.8 and 6.1, respectively; Table 1) compared with LD chops (Δ chroma = 2.0; P < 0.01). No muscle × display day interaction was observed for hue angle (P = 0.19; Figure 3B). Despite the lack of interaction, LD chops had hue angles of at least 10.1 units greater than PM and TB chops (P < 0.01) over the entire display period, indicative of a more yellow hue. A muscle × display day interaction (P < 0.01) was also observed for 630/580 nm ratio (Figure 3C). On day 1 of display, chops from the TB and PM had 630/580 nm ratios that were 1.5 units greater than ratios observed for LD chops (P < 0.01; Figure 3C). However, on day 5 of display, 630/580 nm ratios were only 0.4 greater in TB and PM chops, compared to LD 630/580 nm ratios (P < 0.01). Additionally, overall decreases in 630/580 nm ratio (day 5 − day 1) were greater for TB (1.9 units) and PM (1.9 units) compared to LD chops (0.7 units; P < 0.01). Overall, TB and PM chops had greater changes in chroma and 630/580 nm ratio compared to LD chops, but changes in hue angle did not differ between muscles.
Effect of muscle on instrumental chroma (A), hue angle (B), and 630/580 nm ratio (C) of pork longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) chops over 5 d of retail display. Within a day of display, different letters (a–c) indicate a significant effect of muscle (P < 0.05). Within a muscle, different letters (v–z) indicate a significant effect of day (P < 0.05).
There was a tendency for TB and PM chops to have greater increases in MMb over the display period than LD chops during display (P ≤ 0.10; Figure 4A). On all days of display, MMb was at least 4.9% greater for TB and PM compared with LD chops (P < 0.01), while MMb concentrations of TB and PM chops were not different from each other (P = 0.11) except at day 4 (P < 0.01). Deoxymyoglobin decreased by 4.2 units from day 1 to day 3 for TB chops (P = 0.02), then increased by 14.1% from day 3 to day 5 (P < 0.01; Figure 4B). Psoas major DMb increased by 9.0% during the display period, with the largest increase from day 4 to day 5 (6.1 units; P < 0.01). Alternatively, DMb for the LD did not change over the display period (P ≥ 0.12). The TB had the greatest DMb on all days of display (P < 0.01) except for day 3, while the LD had the lowest DMb on all days of display (P < 0.01), except day 1. A muscle × display day interaction was also observed for OMb (P < 0.01; Figure 4C). The LD had the greatest OMb on all days of display (P < 0.01), while the TB had the lowest OMb on all days (P ≤ 0.01) except day 3, when it was not different from the PM. The TB and PM had greater total (day 5 − day 1) decreases in OMb (29.9 units and 30.4 units, respectively) compared to the LD (20.6 units; P < 0.01), but they did not differ from each other (P = 0.75).
Effect of muscle type on metmyoglobin (A), deoxymyoglobin (B), and oxymyoglobin (C) of pork longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) chops over 5 d of retail display. Within a day of display, different letters (a–c) indicate a significant effect of muscle (P < 0.05). Within a muscle, different letters (v–z) indicate a significant effect of day (P < 0.05).
An interaction between muscle and display day was observed for OC (P = 0.02; Figure 5A), but not for MRA (P = 0.11; Figure 5B). Chops from the LD had decreased OC compared to chops from the TB and PM at all evaluation periods (day 1, day 3, and day 5 of display; P < 0.01). Oxygen consumption for LD chops decreased on each subsequent day of display (P < 0.01), with an overall decrease of 27.5 units from day 1 to day 5 (P < 0.01). Alternatively, OC of TB and PM did not decrease during display (P ≥ 0.10) and were not different from each other at any evaluation period (P ≥ 0.52). Metmyoglobin reducing activity of all muscles decreased during display by 5.6 to 12.6 units on each subsequent evaluation period (P < 0.01). Chops from the LD had at least 5.26 units greater MRA than PM and TB chops at each evaluation period (P < 0.01). Within an individual evaluation period, at day 3 there was a tendency for the TB to have greater MRA than the PM (4.6 units; P = 0.06). At day 5, no difference in MRA was observed between the TB and PM (P = 0.34). There was no muscle × day (P = 0.39) or muscle (P = 0.80) effect for lipid oxidation (Figure 5C). However, lipid oxidation had a slight numerical increase for all muscles from day 1 to day 3 (≥0.07 units; P < 0.01). Overall, TB and PM chops had increased OC and decreased MRA compared to LD chops but did not differ in lipid oxidation. This may have also contributed to increased DMb and decreased OMb on day 1 in TB and PM chops compared to LD chops.
Effect of muscle type on oxygen consumption (A), metmyoglobin reducing activity (B), and lipid oxidation (C) of pork longissimus dorsi (LD), triceps brachii (TB), and psoas major (PM) chops over 5 d of retail display. Within a sampling period, different letters (a–c) indicate a significant effect of muscle (P < 0.05). Within a muscle, different letters (x–z) indicate a significant effect of sampling period (P < 0.05).
Correlation coefficients between overall changes in LD color measurements and overall changes in TB and PM measurements are presented in Table 2. Moderate correlations were observed for the total change in LD and TB redness (r = 0.55; P = 0.01) as well as LD and TB chroma (r = 0.58; P = 0.01). The change in LD OMb was strongly correlated to TB OMb (r = 0.80; P < 0. 01). There was a tendency for correlations between LD and TB hue angle (r = 0.39; P = 0.09) and 630/580 ratio (r = 0.41; P = 0.07). Moderate correlations were observed for the total change in LD and PM lightness (r = 0.57; P = 0.01), redness (r = 0.51; P = 0.02), hue angle (r = 0.63; P < 0.01), and 630/580 nm ratio (r = 0.47; P = 0.04). Yellowness, MMb, DMb, visual discoloration, OC, MRA, and lipid oxidation measurements for the LD were not correlated to like measurements from the TB or PM (P ≥ 0.15).
Correlation coefficients between overall changes during the 5-d display period in longissimus dorsi (LD) color measurements and changes in triceps brachii (TB) or psoas major (PM) color measurements
| TB | PM | |||
|---|---|---|---|---|
| LD Trait | Correlation, r | P Value1 | Correlation, r | P Value1 |
| Δ Lightness, L* | −0.20 | 0.39 | 0.57 | 0.01 |
| Δ Redness, a* | 0.55 | 0.01 | 0.51 | 0.02 |
| Δ Yellowness, b* | 0.33 | 0.16 | 0.08 | 0.75 |
| Δ Chroma2 | 0.58 | 0.01 | 0.30 | 0.19 |
| Δ Hue Angle3 | 0.39 | 0.09 | 0.63 | <0.01 |
| Δ 630/580 nm | 0.41 | 0.07 | 0.47 | 0.04 |
| Δ Metmyoglobin, %4 | 0.26 | 0.27 | 0.34 | 0.15 |
| Δ DMb, %5 | −0.03 | 0.90 | 0.21 | 0.38 |
| Δ OMb, %6 | 0.80 | <0.01 | 0.31 | 0.18 |
| Δ Visual Discoloration, % | −0.21 | 0.37 | −0.24 | 0.31 |
| Oxygen Consumption, % | −0.19 | 0.42 | −0.03 | 0.88 |
| Metmyoglobin Reducing Activity, % | −0.03 | 0.90 | 0.07 | 0.77 |
| mg MDA/kg Meat | 0.3 | 0.20 | 0.24 | 0.31 |
Bold P values indicate significant correlations (P < 0.05).
Chroma is a measure of color saturation, calculated by Hab = arctangent (b*/a*).
Hue angle is a measure of primary color hue of a sample, calculated by Hab = arctangent (b*/a*).
Calculated as follows: .
Calculated as follows: .
Calculated as follows: %OMb = 100 − %MMb − %DMb.
DMb = deoxymyoglobin; MDA = malondialdehyde; MMb = metmyoglobin; OMb = oxymyoglobin.
Discussion
In beef, cuts containing the TB and PM are commonly displayed in overwrap packaging for retail sale, similar to LL chops (Lepper-Bililie et al., 2014). Therefore, the color stability of these muscles in beef has direct implications for retail display. In pork, the TB and PM are less commonly overwrapped for sale. Thus, they were chosen for this study because they represent muscles that are biochemically different from the LD, rather than direct economic implications. Understanding differences in color stability among the TB, PM, and LD would improve understanding of pork discoloration as a whole and provide additional insight into how muscle dependent discoloration differs among species. Furthermore, nearly 20% of pork in the United States is consumed as sausage (Pork Checkoff, 2011), which is produced using several muscles (some of which may have decreased color stability) and pro-oxidant ingredients such as salt. Raines et al. (2010) reported that using muscles with poor color stability reduced color stability of ground beef. As such, color stability of TB and PM may be indicative of color stability for other muscles used for further processed products like sausage. Therefore, there is also economic value in evaluating pork muscles that would be considered color labile in beef, especially if muscle type has a similar impact among species.
Similar to what has been observed in beef, the TB and PM were more color labile during a 5-d display period than the LD. By day 5 of display, 95% of TB chops and 80% of PM chops exceeded 20% visual discoloration, whereas only 15% of LD chops discolored in the same period. Furthermore, the TB and PM had greater decreases in redness, yellowness, chroma, 630/580 nm ratio, and OMb during display than the LD. However, minimal differences between the TB and PM were observed during display. The TB and PM also had higher pH and Mb contents on day 1 of display compared to the LD. Results from the current study were generally in agreement with results observed in beef, as multiple studies have reported beef PM and TB were more color labile than LL (McKenna et al., 2005; Seyfert et al., 2006; Legako et al., 2018; Nair et al., 2018). Seyfert et al. (2006) and Legako et al. (2018) observed beef PM and TB discolored more quickly compared to the LL, whereas McKenna et al. (2005) and Nair et al. (2018) reported greater decreases in redness, yellowness, chroma, hue angle, and 630/580 nm of beef TB and PM compared with LL. Ultimate pH and Mb content, both of which were elevated in color labile beef muscles (McKenna et al., 2005; Jeong et al., 2009), were also greater in pork TB and PM in the current study. In beef, muscles considered “color labile” or “color stable” generally had similar characteristics, such as pH, redness, and OMb content, as other muscles in their stability class (McKenna et al., 2005; King et al., 2011b). Similarities between the color labile TB and PM in the current study suggest that other traditionally color labile muscles in beef like the serratus ventralis, supraspinatus, and bicep femoris may also be color labile in pork.
These similarities between beef and pork discoloration were not unexpected because the rate of accumulation of MMb in meat, which is responsible for changes in meat surface color, is related to fiber types present in a muscle (Ramanathan et al., 2014). In both pork and beef, the PM and TB typically contain a greater proportion of red, oxidative muscle fibers, whereas the LD predominantly contains white, glycolytic fibers (Solomon et al., 1994; Picard and Gagaoua, 2020; Cheng et al., 2021). Oxidative muscle fibers primarily utilize aerobic metabolism to generate energy and therefore contain greater proportions of mitochondria and Mb. The ability of mitochondria to consume oxygen continues postmortem, which can place the muscle tissue under additional oxidative stress and increase Mb oxidation (Ramanathan and Mancini, 2018). As a result, oxidative muscles also generally have an increased OC rate, which is associated with decreased color stability in beef (O’Keefe and Hood, 1982; Renerre and Labas, 1987). Studies have also shown that OC rate was increased in beef TB and PM compared to LL (Mckenna et al., 2005; Mancini et al., 2018). In the current study, the TB and PM both had greater OC than the LD, further supporting the hypothesis that pork color stability differences may be driven by differences in oxygen usage among muscles. Alternatively, glycolytic muscles have enzymes associated with anaerobic metabolism that are able to regenerate NADH, improving the capacity of a muscle to reduce MMb to DMb, mitigating visual formation of discoloration (Joseph et al., 2012; Nair et al., 2018). During display, MRA was reduced in all muscles, but it was consistently greater in LD compared with PM and TB. This is similar to beef in which MRA was increased in the LL compared to TB and PM (McKenna et al., 2005). Furthermore, while absolute MRA for muscle types differed slightly between the current study and McKenna et al. (2005), the pattern of difference between muscles was similar. The difference in MRA between the LL and TB (2.90% in beef, 8.36% in pork) was smaller than the difference between the LL and PM (17.9% in beef, 13.37% in pork). Similar to beef, differences in OC and MRA appear to underlie differences in color stability between pork muscles.
While the TB and PM had greater overall MMb content compared to the LD in the current study, muscles did not differ in rate of MMb accumulation during display. Furthermore, no samples for any muscle reached 20% visual discoloration until day 4, whereas the average MMb for the TB and PM exceeded 20% on day 2 of display. This was unexpected, as studies in beef have reported that muscles with decreased color stability accumulate MMb more quickly. McKenna et al. (2005) observed, in beef, that MMb concentration of TB and PM increased more rapidly than LL. Additionally, previous research in beef has suggested that consumers may begin to discriminate against discoloration when MMb reaches 20% (Renerre and Labas, 1987; Gill and Jones, 1994). This prior research served as the basis for the 20% visual discoloration–consumer acceptability threshold used in this study, since surface discoloration has been used as an approximation for MMb formation (Boler et al., 2009; King et al., 2023). However, in the present study, no samples for any muscle reached 20% visual discoloration until day 4, whereas the average MMb for the TB and PM exceeded 20% on day 2 of display. This may indicate that MMb content alone is not sufficient for estimation of visual discoloration in pork, especially when evaluating multiple muscles. However, in the current study, changes in 630/580 nm ratio and OMb content during display more closely reflected changes in visual discoloration. For example, a large decline in both 630/580 nm ratio and OMb were observed on day 4 and day 5 for the TB and PM, respectively, corresponding to large increases in overall visual discoloration score for those muscles on the same days. Overall, it may be preferred to use additional measures of discoloration beyond MMb content, such as 630/580 nm ratio or OMb content, when evaluating color stability of pork muscles.
Despite differences in color stability, muscles did not differ in lipid oxidation on any day of analysis in the current study. Lipid oxidation and visual discoloration are understood to be related, in beef, with the two processes exacerbating each other (Faustman et al., 2010). However, multiple studies have indicated that pork Mb behaves differently in the presence of lipid oxidation products than beef Mb (Suman et al., 2006). Lipid oxidation products like 4-hydroxy-2-nonenal preferentially adduct to the proximal histidine in beef Mb, destabilizing the molecule and making it more susceptible to oxidation. Alternatively, lipid oxidation products adduct to different sites on pork Mb than beef, causing pork Mb to remain more resistant to protein oxidation even in the presence of lipid oxidation (Suman et al., 2006). Therefore, differences in color stability in the current study were likely not related to lipid oxidation.
One of the objectives of the current study was to determine whether changes in LD color measurements over the 5-d display period were correlated to changes in PM or TB color measurements. If changes in LD color stability traits were related to similar changes in other muscles, this could suggest that genetic selection of improved LD color stability may also improve color stability of other pork muscles as well. In the present study, changes in LD redness, hue angle, and 630/580 were moderately correlated or trending toward a moderate correlation with the same traits in PM or TB. Similarly, King et al (2011b) reported that beef LL color measurements on a given display day were correlated to measurements in other muscles, including the TB. Redness and hue angle for the LL were also correlated to TB measurements on the same display day in the study by King et al. (2011b). In the same study, LL lightness was correlated with TB lightness on days of retail display. In comparison, for the current study, no lightness correlations were observed between muscles. Differences in lightness during display for the current study were relatively small for all muscles, which may explain the lack of correlations. In both King et al. (2011b) and the present study, no correlations for yellowness were observed between the LL or LD and TB. Other than a strong correlation between the LL and TB for OMb, no correlations were observed between muscles for changes in Mb forms in the current study. Hood (1980) reported that MMb content was correlated between the LL and PM in beef. However, differences in MMb calculation method may explain the contrasting findings to the current study. Furthermore, in the present study both MMb and DMb were calculated using multiple reflectance values; this may have increased overall measurement variability and decreased correlations as a result. Changes in LD OMb were correlated to changes in OMb of TB, but not PM. This may indicate that changes in OMb content over time are more variable in the PM. Changes in LD visual discoloration were not predictive of changes in the TB or PM. However, total change (day 5 − day 1) visual discoloration of the LD during display was smaller than TB, resulting in less variability and smaller r-values (Goodwin and Leech, 2006). Despite the smaller change in LD discoloration, correlations were still observed between LD and other muscles for instrumental redness and brownness. Overall, these data would indicate a moderate relationship between the LD and other muscles for instrumental color traits, especially measures related to redness or brownness. Therefore, changes in pork LD color stability may be indicative of color stability changes for other pork muscles as well.
Conclusion
Overall, muscle type affected the rate of discoloration in this study. Differences in discoloration between the pork LD, TB, and PM were similar to color stability differences for the same muscles in beef. The majority of TB and PM samples were discolored by the day 5 of display, whereas 85% of LD samples were still considered acceptable at day 5. Furthermore, the TB and PM had greater total changes in most color traits compared to LD but were similar to each other. Changes in instrumental color were related between the LD and muscles, especially measures of redness or brownness. Based on the relationships between these traits, color stability of the TB and PM may be improved by genetic selection for traits to improve color stability of the LD. While further research is necessary to evaluate color stability differences in other pork muscles, selecting for improved color stability in the LD could improve color stability of an entire carcass, and not just specific muscles.
Literature Cited
Boler, D. D., A. C. Dilger, B. S. Bidner, S. N. Carr, J. M. Eggert, J. W. Day, M. Ellis, F. K. McKeith, and J. Killefer. 2009. Ultimate pH explains variation in pork quality traits. J. Muscle Foods 21:119–130. doi: https://doi.org/10.1111/j.1745-4573.2009.00171.x.
Cheng, H., S. Song, and G. D. Kim. 2021. Frozen/thawed meat quality associated with muscle fiber characteristics of porcine longissimus thoracis et lumborum, psoas major, semimembranosus, and semitendinosus muscles. Sci. Rep.-UK 11:13354. doi: https://doi.org/10.1038/s41598-021-92908-3.
CIE (Commission Internationale de l’Eclairage). 1976. Colorimetry–Part 4: CIE 1976 L*a*b* colour space. CIE Central Bureau, Vienna, Austria.
Faustman, C., and A. Phillips. 2001. Measurement of discoloration in fresh meat. Current Protocols in Food Analytical Chemistry F3.3.1–F3.3.13. doi: https://doi.org/10.1002/0471142913.faf0303s00.
Faustman, C., Q. Sun, R. Mancini, and S. P. Suman. 2010. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 86:86–94. doi: https://doi.org/10.1016/j.meatsci.2010.04.025.
Font-i-Furnols, M., and L. Guerrero. 2014. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 98:361–371. doi: https://doi.org/10.1016/j.meatsci.2014.06.025.
Gill, C. O., and T. Jones. 1994. The display life of retail-packaged beef steaks after their storage in master packs under various atmospheres. Meat Sci. 38:385–396. doi: https://doi.org/10.1016/0309-1740(94)90065-5.
Goodwin, L. D., and N. L. Leech. 2006. Understanding correlation: factors that affect the size of r. J. Exp. Educ. 74:249–266. doi: https://doi.org/10.3200/JEXE.74.3.249-266.
Hood, D. E. 1980. Factors affecting the rate of metmyoglobin accumulation in pre-packaged beef. Meat Sci. 4:247–265. doi: https://doi.org/10.1016/0309-1740(80)90026-1.
Jeong, J. Y., S. J. Hur, H. S. Yang, S. H. Moon, Y. H. Hwang, G. B. Park, and S. T. Joo. 2009. Discoloration characteristics of 3 major muscles from cattle during cold storage. J. Food Sci. 74:C1–C5. doi: https://doi.org/10.1111/j.1750-3841.2008.009.
Joseph, P., S. P. Suman, G. Rentfrow, S. Li, and C. M. Beach. 2012. Proteomics of muscle-specific beef color stability. J. Agric. Food Chem. 60:3196–3203. doi: https://doi.org/dx.doi.org/10.1021/jf204188v.
King, D. A., M. C. Hunt, S. Barbut, J. R. Claus, D. P. Cornforth, P. Joseph, Y. H. B. Kim, G. Lindahl, R. A. Mancini, M. N. Nair, K. J. Merok, A. Milkowski, A. Mohan, F. Pohlman, R. Ramanathan, C. R. Raines, M. Seyfert, O. Sørheim, S. P. Suman, and M. Weber. 2023. American Meat Science Association guidelines for meat color measurement. 6:1–81. doi: https://doi.org/10.22175/mmb.12473.
King, D. A., S. D. Shackelford, and T. L. Wheeler. 2011a. Use of visible and near-infrared spectroscopy to predict pork longissimus lean color stability. J. Anim. Sci. 89:4195–4206. doi: https://doi.org/10.2527/jas.2011-4132.
King, D. A., S. D. Shackelford, and T. L. Wheeler. 2011b. Relative contributions of animal and muscle effects to variation in beef lean color stability. J. Anim. Sci. 89:1434–1451. doi: https://doi.org/10.2527/jas.2010-3595.
Krzywicki, K. 1979. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. doi: https://doi.org/10.1016/0309-1740(79)90019-6.
Legako, J. F., T. Cramer, K. Yardley, T. J. Murphy, T. Gardner, A. Chail, L. Pitcher, and J. W. MacAdam. 2018. Retail stability of three beef muscles from grass-, legume- and feedlot- finished cattle. J. Anim. Sci. 96:2238–2248. doi: https://doi.org/10.1093/jas/sky125.
Leick, C. M., C. L. Puls, M. Ellis, J. Killefer, T. R. Carr, S. M. Scramlin, M. B. England, A. M. Gaines, B. F. Wolter, S. N. Carr, and F. K. McKeith. 2010. Effect of distillers dried grains with solubles and ractopamine (Paylean) on quality and shelf-life of fresh pork and bacon. J. Anim. Sci. 88:2751–2766. doi: https://doi.org/10.2527/jas.2009-2472.
Lepper-Bililie, A. N., E. P. Berg, A. J. Germolus, D. S. Buchanan, and P. T. Berg. 2014. Consumer evaluation of palatability characteristics of a beef value-added cut compared to common retail cuts. Meat Sci. 96:419–422. doi: https://doi.org/10.1016/j.meatsci.2013.08.002.
Mancini, R. A., and M. C. Hunt. 2005. Current research in meat color. Meat Sci. 71:100–121. doi: https://doi.org/10.1016/j.meatsci.2005.03.003.
Mancini, R. A., K. Belskie, S. P. Suman, and R. Ramanathan. 2018. Muscle-specific mitochondrial functionality and its influence on fresh beef color stability. J. Food Sci. 83:2077–2082. doi: https://doi.org/10.1111/1750-3841.14219.
McKenna, D. R., P. D. Mies, B. E. Baird, K. D. Pfeiffer, J. W. Ellebracht, and J. W. Savell. 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682. doi: https://doi.org/10.1016/j.meatsci.2005.02.016.
Nair, M. N., S. Li, C. M. Beach, G. Rentfrow, and S. P. Suman. 2018. Changes in the sarcoplasmic proteome of beef muscles with differential color stability during postmortem aging. Meat Muscle Biol. 2:1–17. doi: https://doi.org/10.22175/mmb2017.07.0037.
North American Meat Processors Association (NAMP). 2014. The Meat Buyer’s Guide. 8th edition. North Am. Meat Institute, Reston, VA.
O’Keefe, M., and D. E. Hood. 1982. Biochemical factors influencing metmyoglobin formation on beef from muscles of differing colour stability. Meat Sci. 7:209–228. doi: https://doi.org/10.1016/0309-1740(82)90087-0.
Pork Checkoff. 2011. Quick facts: the pork industry at a glance. Pork Checkoff, National Pork Board, Des Moines, IA.
Picard, B., and M. Gagaoua. 2020. Muscle fiber properties in cattle and their relationship with meat qualities: An overview. J. Agric. Food Chem. 68:6021–6039. doi: https://doi.org/10.1021/acs.jafc.0c02086.
Ramanathan, R., S. P. Suman, and C. Faustman. 2014. Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: a review. J. Agric. Food Chem. 68:12779–12787. doi: https://doi.org/10.1021/acs.jafc.9b08098.
Ramanathan, R., and R. A. Mancini. 2018. Role of mitochondria in beef color: A review. Meat Muscle Biol. 2:309–320. doi: https://doi.org/10.22175/mmb2018.05.0013.
Raines, C. R., M. C. Hunt, and J. A. Unruh. 2010. Contributions of muscles of various color stabilities to the overall color stability of ground beef. J. Food Sci. 75:C85–C89. doi: https://doi.org/10.1111/j.1750-3841.2009.01430.x.
Renerre, M., and R. Labas. 1987. Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Sci. 19:151–165. doi: https://doi.org/10.1016/0309-1740(87)90020-9.
Seyfert, M., R. A. Mancini, M. C. Hunt, J. Tang, C. Faustman, and M. Garcia. 2006. Color stability, reducing activity, and cytochrome c oxidase activity of five bovine muscles. J. Agric. Food Chem. 54:8919–8925. doi: https://doi.org/10.1021/jf061657s.
Solomon, M. B., T. J. Caperna, R. J. Mroz, and N. C. Steele. 1994. Influence of dietary protein and recombinant porcine somatotropin administration in young pigs: III. Muscle fiber morphology and shear force. J. Anim. Sci. 72:615–621. doi: https://doi.org/10.2527/1994.723615x.
Souza, C. M., D. D. Boler, D. L. Clark, L. W. Kutzler, S. F. Holmer, J. W. Summerfield, J. E. Cannon, N. R. Smit, F. K. McKeith, and J. Killefer. 2011. The effects of high pressure processing on pork quality, palatability, and further processed products. Meat Sci. 87:419–427. doi: https://doi.org/10.1016/j.meatsci.2010.11.023.
Suman, S. P., C. Faustman, S. L. Stamer, and D. C. Liebler. 2006. Redox instability induced by 4-hydroxy-2-nonenal in porcine and bovine myoglobins at pH 5.6 and 4°C. J. Agric. Food Chem. 54:3402–3408. doi: https://doi.org/10.1021/jf052811y.
Taylor, R. 1990. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonor. 6:35–39. doi: https://doi.org/10.1177/875647939000600106.
Zhu, L. G., and M. S. Brewer. 1998. Discoloration of fresh pork as related to muscle and display conditions. J. Food Sci. 63:763–767. doi: https://doi.org/10.1111/j.1365-2621.1998.tb17895.x.