Introduction
Fatty acid composition in meat is a topic of interest to both the scientific community and the public. Fat in meat animals consists mostly of triglycerides, acquired from dietary sources and fatty acid de novo synthesis (Dinh et al., 2010; Bravo-Lamas et al., 2018). Fatty acids are classified by the length of their carbon chains and the number of double bonds. The interest in fatty acids is related to nutritional and sensory implications. Fatty acid composition and the roles of each fatty acid in thermal oxidation during cooking are of interest because of their contribution to the cooked meat aromas (Khan et al., 2015; Arshad et al., 2018). The thermal oxidation of both saturated fatty acids (SFA) and unsaturated fatty acids creates classes of compounds (i.e., alkanes, aldehydes, ketones, organic acids, etc.) similar to those that are formed during lipid autoxidation (Khan et al., 2015; Arshad et al., 2018; Dinh et al., 2018). However, thermal oxidation produces much more desirable aromas because it shifts the composition of the oxidation products toward a greater concentration of acids, esters, long-chain aldehydes and ketones, and various polymerized compounds. Many reviews have discussed meat flavor, including various pathways of flavor compounds from both fat- and water-soluble fractions (Calkins and Hodgen, 2007; Arshad et al., 2018; Bravo-Lamas et al., 2018; Dinh et al., 2018). The objective of this review, however, is to provide an overview of fatty acids in meat, their variation in meat from various species, and their roles as meat flavor precursors during cooking.
Overview of Fatty Acids in Meat
Animal fat is commonly classified as depot fat (localized in adipose tissues) and intramuscular fat (localized in muscle tissues; Rhee et al., 2000). Depot fats are mostly localized in the subcutaneous layer, although they also include intermuscular deposits (seam fats). Intramuscular fat includes lipids from adipose cells in the muscle (marbling) and membrane-bound lipids. However, in red meat, the term “intramuscular fat” normally denotes marbling. In fresh red meat, the principal lipid components of depot fats are triglycerides, which can be easily extracted using non-polar organic solvents. Slightly different from subcutaneous fats, marbling contains more phospholipids, which are associated with proteins as lipoproteins or proteolipids (Rhee et al., 2000; Aberle et al., 2001). Although triglycerides are still predominant in marbling, in very lean meat, the cellular phospholipids may account for up to one-third of fat content (Rhee et al., 2000). The fatty acids found in triglycerides and other lipids in red meat are differentiated by the carbon chain length and the type of bonding between carbons. Fatty acids are carboxylic acids, typically composed of hydrocarbon chains with a methyl group on one end and a carboxyl group on the other (Figure 1). Fatty acids in meats are usually unbranched and have an even number of carbons from 4 to 24, although 12 to 24 is most common (Voet et al., 2006). However, some branched or odd-numbered fatty acids can be found in ruminant adipose tissues or milk fat (Lobb and Chow, 2000; Duncan, 2001) because of propionate production in the rumen.
Typical features of fatty acids (ACD/ChemSketch, 2020).
Fatty acids in foods are most often esterified, monocarboxylic acids, which are classified as SFA (no double bond), monounsaturated fatty acids (MUFA) (1 double bond), or polyunsaturated fatty acids (PUFA) (2 or more double bonds; Figure 2). The 4 common systems to name fatty acids are common names, International Union of Pure and Applied Chemistry (IUPAC) names, carboxyl-reference, and omega-reference abbreviated names as shown in Table 1 (Duncan, 2001). Common names are derived from the primary source of specific fatty acids, such as olive oil and oleic acid, and contain no structural information. The other nomenclature systems are used to identify the chain length and number and position of double bonds so that the structures of fatty acids can be determined. The IUPAC system denotes the carboxyl carbon as the first carbon and other carbons as a reference to the carboxyl group. The carbon chain is named according to the saturated hydrocarbon with the suffix “-anoic” for SFA and “-enoic” for MUFA and PUFA. The numbers are used to identify the position of the double bond, and “cis” and “trans” are used to describe the three-dimensional configuration. The carboxyl-reference system is different from the IUPAC system in using numbers to name the carbon chain, whereas the omega-reference system is used to indicate the position of the double bond that is closest to the omega carbon, i.e., the last (methyl) carbon. This system is useful for physiological consideration between ω-3 and ω-6 fatty acids. The omega symbol can be replaced with “n.”
Typical 18-carbon fatty acids with different degrees of saturation (ACD/ChemSketch, 2020).
Fatty acid nomenclature systems
| Common | IUPAC | Carboxyl Reference | ω Reference |
|---|---|---|---|
| Palmitic acid | Hexadecanoic acid | 16:0 | 16:0 |
| Stearic acid | Octadecanoic acid | 18:0 | 18:0 |
| Oleic acid | cis9-octadecanoic acid | 18:1 Δ9 | 18:1 ω9 |
| Linoleic acid | cis9,12-octadecanoic acid | 18:2 Δ9,12 | 18:2 ω6 |
| Linolenic acid | cis 9,12,15-octadecanoic acid | 18:3 Δ9,12,15 | 18:3 ω3 |
IUPAC = International Union of Pure and Applied Chemistry.
The presence of a double bond allows for the occurrence of 2 different geometric configurations: “cis” and “trans” (Figure 3). The cis configuration is formed when both hydrogen atoms of the two carbons joined by a double bond are on the same planar side of the double bond. The cis configuration is the most common naturally occurring configuration and possesses a distinctly different geometry from SFA (Lobb and Chow, 2000). In contrast, the trans configuration does not change the molecule shape, and it is very similar to the SFA configuration. Trans configuration naturally occurs in ruminant meat and milk in small concentrations. The major fatty acids in red meat are myristic (14:0), palmitic (16:0), and stearic (18:0) acids in the SFA category; palmitoleic (16:1) and oleic (18:1) acids in the MUFA category; and linoleic (18:2), linolenic (18:3), and arachidonic (20:4) acids in the PUFA category (Table 2).
Structural differences between cis- and trans-configuration of 18:1Δ9 fatty acids (ACD/ChemSketch, 2020).
Percentages of major fatty acids in red meats
| Product* | SFA | MUFA | PUFA | 14:0 | 16:0 | 18:0 | 16:1 | 18:1 | 18:2 | 18:3 | 20:4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beef | |||||||||||
| Concentrate finishing | 40.9–47.9 | 40.1–49.9 | 2.9–10.7 | 2.0–3.7 | 23.8–28.0 | 12.8–14.8 | 3.6–5.7 | 30.5–44.5 | 2.5–7.7 | 0.1–0.9 | 0.2–3.5 |
| Grass finishing | 47.7- 51.5 | 25.4–47.2 | 3.6–7.9 | 2.5–3.0 | 28.3–30.7 | 14.2–18.0 | 2.5–3.5 | 31.3–42.8 | 2.12–3.1 | 0.7–1.7 | 0.3–0.8 |
| Lamb | 40.8–53.3 | 37.9–47.3 | 3.6–8.6 | 1.9–4.0 | 20.9–28.8 | 16.1–23.9 | 1.4–2.2 | 35.7–54.3 | 3.2–5.2 | 0.6–2.20 | 0.5–1.1 |
| Goat | 37.2–53.8 | 33.7–53.9 | 8.9–16.5 | 1.6–5.1 | 17.3–31.4 | 15.0–17.3 | 2.1–5.7 | 28.0–49.2 | 5.2–11.8 | 1.1–1.2 | 3.1–4.7 |
| Pork | 36.9–41.7 | 26.9–46.8 | 14.0–32.5 | 1.1–1.5 | 23.8–26.7 | 11.9–14.0 | 2.5–3.6 | 31.1–42.8 | 11.3–23.3 | 0.2–0.4 | 1.4–6.5 |
| Horse | 35.8–36.8 | 27.4–34.6 | 29.7–35.8 | 2.3–4.5 | 24.2–25.9 | 5.5–7.5 | 2.9–4.5 | 22.2–28.1 | 11.7–22.9 | 7.9–16.6 | 0.7–3.0 |
Beef: Banskalieva et al., 2000; Leheska et al., 2008; USDA, 2021 (loin, lean only, raw; FDC ID: 173970, FDC ID: 174002). Lamb: Banskalieva et al., 2000; USDA, 2021 (loin chop, lean only, raw; FDC ID: 173810). Goat: Banskalieva et al., 2000; USDA, 2021 (game meat, raw; FDC ID: 175303). Pork: Banskalieva et al., 2000; Yu and Shu, 2013; USDA, 2021 (center loin [chops], lean only, raw; FDC ID: 168263). Horse: Tonial et al., 2009; Ferjak et al., 2019.
MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; SFA = saturated fatty acids.
Among SFA, palmitic and stearic acids occur most predominantly (Lobb and Chow, 2000). These fatty acids are found in fish oil, milk, body fats of land animals, and almost all vegetable oils at levels between 5% and 50% of total fatty acids (Jenkins, 1993). Stearic acid is more likely to be found in animal fat than other sources because of the elongation of palmityl-coenzyme A and biohydrogenation in the rumen (Jenkins, 1993). Most MUFA have the cis configuration at Δ9 or ω9 (Gunstone, 1996). Oleic acid (18:1 n-9cis) is present in significant concentrations in many foodstuffs, specifically in animal fats because it serves as the biological precursor of other n-9 monoene fatty acids and the n-9 family of polyene acids (Gunstone, 1996). The most common PUFA in meats have a methylene-interrupted double bond system with 2 to 6 double bonds and the cis configuration (Gunstone, 1996). Two major groups of PUFA are n-6 fatty acids based on linoleic acid and the n-3 group based on α-linolenic acid. The most common C18 PUFA in meats—i.e., linoleic and linolenic acids—are also found in vegetable oils (Wallis et al., 2002), whereas arachidonic acid (20:4 n-6) is one of the few important PUFA only present in animal fats (Gunstone, 2012). In addition to the normal methylene interrupted double bond system, which is most abundant in the PUFA structure, conjugated or polymethylene interrupted double bonds also exist in meat. The conjugated diene system in the tissue of ruminant animals has garnered attention because of its human health benefits (Schmid et al., 2006). Polyunsaturated fatty acids such as linolenic acid are modified by ruminal microorganisms to form conjugated linoleic acids—which are intermediates or byproducts of biohydrogenation—with 18:2 cis-9 trans-11 and 18:2 trans-10 cis-12 as the main isomers (Wahle et al., 2004; Schmid et al., 2006; Sébédio and Ratnayake, 2008).
Ruminant Fat
Ruminant fatty acid composition is greatly influenced by biohydrogenation in the rumen, which saturates linoleic and linolenic acids, as a protective mechanism from natural microflora against the toxic effects of unsaturated fatty acids (Wood et al., 2008; Shingfield et al., 2013; Bessa et al., 2015); therefore, even though PUFA are the predominant fatty acids in cattle feeds (both forage and concentrated diets; Palmquist and Jenkins, 1980; French et al., 2000; Nuernberg et al., 2005; Warren et al., 2008; Fruet et al., 2018), the triglyceride composition of beef contains much more SFA and MUFA than PUFA. However, the percentage of PUFA can be increased because PUFA—if fed at a high concentration such as through the inclusion of forage or distillers grains—will bypass the rumen. Reviews on the effects of diets on ruminant fat and fatty acid metabolism suggest that inclusion of forage and distillers grains in ruminant diets increases the passage of 18:2 and 18:3 (Klopfenstein et al., 2008; Schingoethe et al., 2009) and the tissue percentage of these fatty acids as well as total PUFA (Wood et al., 2008; Kouba and Mourot, 2011; Bessa et al., 2015; Brzozowska and Oprządek, 2016). Although an overall increase in PUFA may facilitate the deposition of more PUFA in the neutral lipid fraction (Warren et al., 2008), the percentage of PUFA is much greater in the cell membrane, which is detected in the phospholipid fraction. The unsaturation of phospholipids is the major cause of off-flavors and oxidative rancidity in meat (Legako et al., 2015). Contrary to common belief, beef fats have more unsaturated fatty acids in edible portions, according to the USDA FoodData Central (FDC) (USDA, 2021), which reports the fatty acid composition of thousands of meat products. Averaged across all quality grades (product #23369: beef, loin, top loin steak, boneless, lip off, separable lean only, trimmed to 0” fat, all grades, raw), beef lean has 5.7 g of fat in 100 g of separable lean (5.7%). This ranges from 3% in USDA Standard to 12% in USDA Prime beef (Legako et al., 2015). Beef marbling typically comprises 43% SFA, 50% MUFA, and 7% PUFA (USDA, 2021). The major SFA—palmitic and stearic acids—constitute 25% and 13%, respectively, of the total fatty acids. In the MUFA category, oleic acid alone contributes 40% to the total fatty acids. Linoleic acid accounts for more than 5% of the total fatty acids. An important animal-origin fatty acid, arachidonic acid, constitutes only 0.8% of the fatty acid composition in beef. As marbling increases, the concentration (milligrams per gram of muscle) of almost all fatty acids in beef also increases; however, this increase is not the same for all fatty acids because SFA and MUFA are deposited in adipose tissues at a greater rate than PUFA (Dinh et al., 2010). Dinh et al. (2010) reported that SFA and MUFA were more correlated with intramuscular fat content than PUFA. In a review by De Smet et al. (2004), both SFA and MUFA content (milligrams/gram) linearly increased with intramuscular content, whereas PUFA content remained constant, confirming what was reported by Dinh et al. (2010). The authors also summarized an inverse relationship between PUFA/SFA ratio and intramuscular fat content. This phenomenon has been found across all livestock species because lipids stored in adipose tissues are usually neutral lipids rich in triglycerides, which in turn contain more SFA and MUFA than PUFA in their structure (Brockerhoff et al., 1966). De Smet et al. (2004) reported that triglycerides in animal fats tend to have PUFA in position 2, whereas palmitic and oleic acids occupy positions 1 and 3 of the triglyceride structure. Moreover, C16 and C18 SFA and MUFA are natural end-products of fatty acid de novo synthesis (De Smet et al., 2004). Legako et al. (2015) substantiated these findings with data revealing a linear increase in concentrations of fatty acids in the neutral lipid fraction as total fatty acids increased, whereas concentrations of fatty acids in the polar lipid fraction remained unchanged.
In beef and other ruminant meats, biohydrogenation in the rumen contributes to the saturation of fatty acids; therefore, the meats from other ruminants have a very similar fatty acid composition to that of beef. Banskalieva et al. (2000) reported in a review paper that goat meat is typically very lean—with 2% to 3% intramuscular fat unless the animals are specifically fattened—regardless of anatomical location, sex, breed, and dietary influences. Across various meat cuts, goat meat has 30% to 40% SFA, more than 50% MUFA, and less than 10% PUFA. The authors reported 10% to 12% PUFA in only a few studies. In the USDA FDC, a goat product classified as game meat (FDC ID 175303; game meat, goat, raw) has only 37% SFA and 9% PUFA. The game meat designation may indicate that wild goat meat is represented in the database, which explains a high PUFA percentage. Our previous research showed that feeding castrated male Kiko goats with deoiled distillers dried grains with solubles led to a decrease in PUFA concentration in the fat. The goat meat had 44% SFA, 54% MUFA, and only 2% PUFA (Camareno et al., 2016). A similar phenomenon of the shifting between SFA and PUFA was reported by Brassard et al. (2017) on concentrate-fed Boer kids. The goat muscle had 40% SFA, 54% MUFA, and 6% PUFA. These findings again indicate that PUFA can bypass the rumen and, if naturally occurring (forage) or supplemented (distillers grain) more in feeds, will be deposited more in the adipose tissues. The MUFA seems to be unaffected, fluctuating in a narrow range of 50% to 54%. Interestingly, in beef cattle, grass-finished beef has up to 4% less MUFA than grain-finished beef (Leheska et al., 2008). This decrease was translated into an increase in SFA and PUFA proportions (Leheska et al., 2008; Daley et al., 2010) because these two categories become more predominant in the total fatty acid composition.
The fatty acid composition of lamb includes 42% SFA, 47% MUFA, and 11% PUFA (USDA, 2021; FDC ID: 174307; lamb, composite of trimmed retail cuts, separable lean only, trimmed to 1/4” fat, choice, raw), although antemortem factors such as sex, breed, and diet also influence lamb fatty acid composition (Ribeiro et al., 2011). The predominant fatty acids in SFA, MUFA, and PUFA categories in lamb are the same as in beef, although the percentages may vary up to 5%. The inclusion of more PUFA in the diets also increased PUFA percentage in lamb, similar to other ruminant species. These compositional data agree with various published data in the literature (Bravo-Lamas et al., 2016; Oliveira et al., 2017). Compared with beef, lamb has up to 5% more SFA, less MUFA, and approximately 0.5% greater arachidonic acid. This is significant for flavor development because thermal oxidation of MUFA contributes to the formation of a different, more desirable flavor profile than SFA and PUFA. In addition, the autoxidation of arachidonic acid is one of the primary sources of off-odors in meat (Frankel, 1980; Pegg and Shahidi, 2012). Lamb has up to 3% volatile branched-chain fatty acids (Bravo-Lamas et al., 2016, 2018), which are precursors of characteristic lamb and mutton odors. These odor-producing fatty acids concentrate more in subcutaneous fat than in muscle (Brennand and Lindsay, 1992). Longer branched-chain fatty acids (14–16 carbons) have been found in beef (unpublished data); however, they do not contribute to characteristic odors in beef.
Monogastric Fat
Monogastric animals such as pigs cannot hydrogenate fatty acids; therefore, their muscle fatty acids are more similar to the fatty acid composition of their diets, as influenced by fatty acid synthesis, elongation, Δ9-desaturation, and deposition. On average, pork has 38% SFA, 50% MUFA, and 12% PUFA (USDA, 2021; FDC ID: 168251; pork, fresh, loin, top loin [chops], boneless, separable lean only, raw). In the SFA category, palmitic and stearic acids account for 25% and 12% of the total fatty acids, respectively. Oleic acid in pork fat is slightly more than that in beef fat, contributing 45% to the total fatty acids. Linoleic acid constitutes approximately 10% of the total fatty acids. The arachidonic acid proportion in pork fat is slightly greater than that in beef fat, at 1.4%. The contribution of each fatty acid to flavor development may not be different among livestock species, except for the fatty acids that provide distinct species odors. However, the fatty acid composition of pork can be easily manipulated by altering the dietary composition, such as fatty acids (Jiang et al., 2017; Komprda et al., 2020) or amino acids (Wang et al., 2018). By supplementing the pigs’ diet with soybean oil and linseed oil, Jiang et al. (2017) were able to decrease SFA by 6% to 7% and MUFA by 12% but increase PUFA by more than 14% in comparison with the data from the USDA FDC. de Tonnac et al. (2018) reported similar findings with pigs that were fed microalgae rich in n-3 fatty acids and contained less SFA and MUFA in muscle but more n-3 fatty acids. Interestingly, n-3 fatty acid deposition varied by fat depots. This depot-specific phenomenon has been found in other monogastric species such as horses, a monogastric species which grazes on forage rich in linoleic and linolenic acids (Belaunzaran et al., 2017). Wang et al. (2018) altered the lysine content in the diet of pigs and were able to change oleic acid in intramuscular fat by approximately 3%. The authors observed that the activity of stearoyl-coenzyme A desaturase was increased with a lysine-deficient diet. This enzyme desaturates stearic acid to produce more oleic acid in muscle tissues.
Although horse meat is rarely consumed in the US, it is a popular meat in various countries around the world. In one of the very few studies on the fatty acid composition of horse meat, Belaunzaran et al. (2017) reported 36% to 37% SFA, 32% to 35% MUFA, and 23% to 27% PUFA. These findings are similar to 35% to 37% SFA, 35% to 37% MUFA, and 26% to 30% PUFA in our study on stock-type horse subcutaneous and intermuscular adipose tissues (Ferjak et al., 2019). Moreover, similar to lamb, horse meat has approximately 0.4% branch-chained fatty acids. The predominant fatty acids in the SFA and MUFA categories are the same as other species. However, the predominant PUFA is linolenic acid at 17%, in comparison with 12% linoleic acid. This reflects the fact that linolenic acid is the predominant fatty acid in most grasses (Palmquist and Jenkins, 1980; Khan et al., 2012) and that horse is a monogastric animal typically grazing on grasses. However, grasses only have 3.4% oleic acid, which reveals the significant role of fatty acid de novo synthesis, including elongation and Δ9-desaturation in livestock adipose tissues since oleic acid is still one of the predominant fatty acids in horse meat. The distribution of fatty acids, especially unsaturated fatty acids, vary by tissue location. Abdominal fat is most saturated (37%–38%), whereas cardiac fat has more PUFA (37%–38%)—although cardiac fat also has palmitic acid (25%–26%) as the predominant fatty acid instead of oleic acid (22%–24%) as in other fat depots (Ferjak et al., 2019). This phenomenon is similar to what has been reported in pork meat, another monogastric species. A high percentage of PUFA—especially the predominance of linolenic acid (15%–17%)—may contribute to lipid oxidation that contributes to the development of negative flavors in horse meat (Ferjak et al., 2019).
Fatty Acids as Flavor Precursors
Overview of lipid oxidation
Lipid-derived flavor compounds include aldehydes (alkanals), ketones (alkanones), carboxylic acids (alkanoics), alcohols (alkanols), lactones, and alkylfurans (Mottram, 1998). These compounds result from the oxidation of fatty acids in various chain reactions of free radicals. These reactions, if occurring during storage, lead to an undesirable aromatic profile and the end of shelf life (Amaral et al., 2018; Domínguez et al., 2019). However, during cooking, similar reactions under thermal oxidation produce desirable and often characteristic cooked flavor profiles (Nawar, 1984; Song et al., 2011). The lipid autoxidation mechanism has been reviewed extensively by Frankel (1980, 1984, 1991) and Grosch (1982). Recently, the focus has been shifted to the interaction of lipid oxidation products with other biological compounds such as cholesterol (Dinh and Thompson, 2016), proteins (Faustman et al., 2010), and Maillard reaction products (Zamora and Hidalgo, 2011; Zhao et al., 2019) to elucidate the complex impacts of lipid oxidation on the nutritional value, color, and flavor of the meat. Lipid autoxidation and thermal oxidation occur by the same free radical mechanisms through initiation, propagation, and termination processes; however, optimal oxygen concentration (Choe and Min, 2007) and higher temperature during cooking (Wasserman, 1972) produce desirable flavor components in the aroma of cooked meat.
Lipid oxidation starts with the abstraction of allylic hydrogen—the hydrogen at an allylic carbon adjacent to the C=C double bond—on the carbon chain of MUFA or PUFA by a reactive oxygen species, such as •OH radical or singlet oxygen (Figure 4). The initiation produces lipid radicals that propagate through various structural changes, by either addition of oxygen (radical coupling), atom transfer (radicalization of another lipid molecule), fragmentation (decomposition), or rearrangement of double bonds (formation of conjugated dienes). Lipid radicals continue to propagate through the chain reactions until 2 lipid radicals react to form a nonradical compound (Frankel, 1980).
Initiation of lipid oxidation by •OH radical (ACD/ChemSketch, 2020).
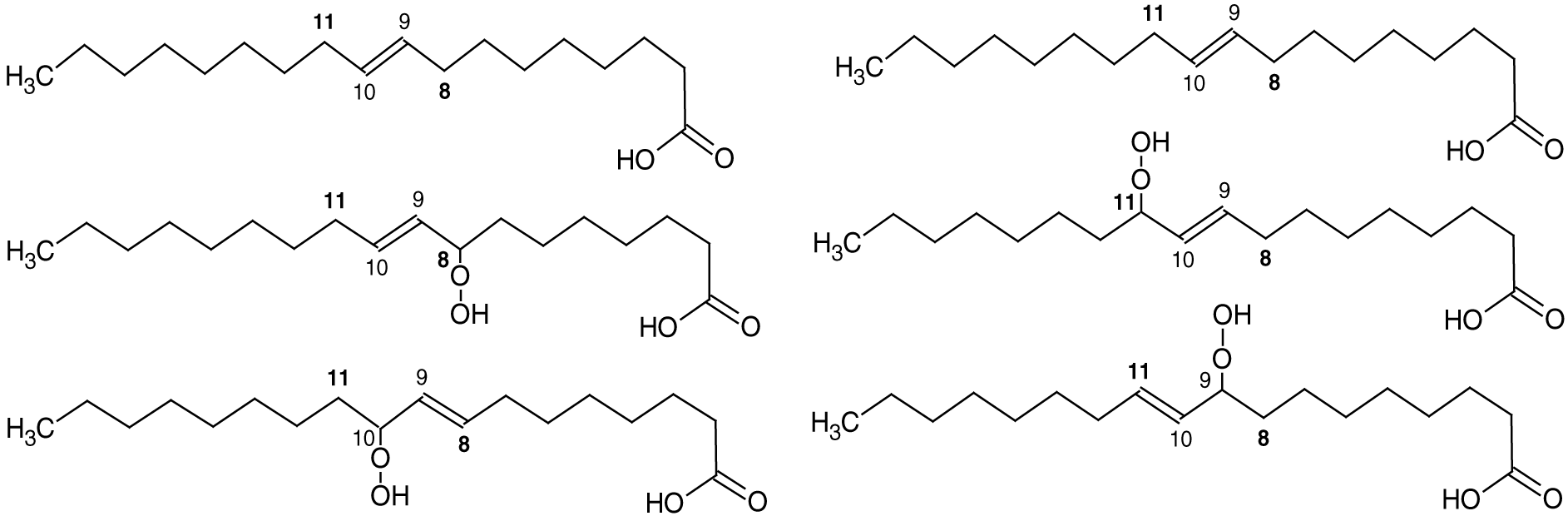
With the availability of oxygen and heat, radical coupling with oxygen is likely to occur in meat products during cooking, which produces hydroperoxides. This leads to various other structural changes, including the rearrangement of double bonds, cyclization, and fragmentation (Figure 5). Hydroperoxides are subsequently decomposed into various volatile compounds that contribute to the cooked meat flavor. The most common MUFA, oleic acid, and the most common PUFA, linoleic acid, in meat produce various hydroperoxides at carbon 8, 9, 10, 11, and 13 (Frankel, 1980; Figures 6–7). The oxygen–oxygen bond of hydroperoxides (R-O-O-H) is relatively weak and therefore not stable during heating (Choe and Min, 2007). Hydroperoxides are decomposed to alkoxy radicals and hydroxy radicals by homolysis of the peroxide bond. The alkoxy radicals are then decomposed or react with other alkoxy radicals to form nonradical volatile or nonvolatile compounds (Choe and Min, 2007) such as aldehydes, esters, alcohols, ketones, carboxylic acids, and hydrocarbons. This process is called the termination of oxidation (Figure 8).
Various mechanisms for the propagation of lipid oxidation (ACD/ChemSketch, 2020; adapted from Frankel, 1980).
Hydroperoxides from oleic acid through abstraction of the allylic hydrogens at carbon 8 or 11 (ACD/ChemSketch, 2020).
Hydroperoxides from linoleic acid through abstraction of the allylic hydrogen at carbon 11 (ACD/ChemSketch, 2020).
Decomposition of hydroperoxides and termination of lipid radicals (ACD/ChemSketch, 2020).
Lipid oxidation products as flavor compounds
The decomposition of oleate hydroperoxides yields decanal, nonanal, octanal, short-chain alcohols (heptanol and octanol), and several carboxylic acids such as octanoic acid. The decomposition of linoleate hydroperoxides yields unsaturated aldehydes and carboxylic acids such as 2- and 3-nonenal and 11-tridecadienoate (Frankel, 1980). Moreover, hundreds of compounds classified as lipid-derived volatiles by Mottram (1998) are formed. These compounds include n-aldehydes, ketones, carboxylic acids, alcohols, and alkanes that have been confirmed in various beef and pork flavor studies (King et al., 1993; Elmore et al., 1999, 2004; Legako et al., 2015; Hunt et al., 2016; Wang et al., 2016; Frank et al., 2017). Song et al. (2011) reported that hexanal, 1-octen-3-ol, (E,E)-2,4-decadienal, and (E,E)-2,4-heptadienal are characteristic of beef flavor in addition to butanoic, 2-methylbutanoic, 3-methylbutanoic, heptanoic, 4-ethyloctanoic, and nonanoic acids (Um et al., 1992). These authors also reported that other n-aldehydes and unsaturated aldehydes such as (E)-2-nonenal, 4-heptenal, nonanal, octanal, and (E)-2-decenal contribute to undesirable cooked beef flavor. Wang et al. (2016) reported slight differences in pork-characteristic volatiles, including ethyl acetate, 3-(methylthio) propanal, hexanal, 2-butanone, dimethyl disulfide, and dimethyl trisulfide, among which 2-butanone and dimethyl disulfide were also found in beef volatiles (Legako et al., 2015; Hunt et al., 2016). Lamb-characteristic volatiles result from alkylpyrazines, such as 2,5-dimethylpyrazine, and alkylpyridines, such as 2-pentylpyridine (Buttery et al., 1977); both are products of reactions between lipid oxidation products and Maillard reaction products. Lamb volatiles also contain more saturated aldehydes than goat meat and other meats (Buttery et al., 1977; Mottram, 1998; Madruga et al., 2013).
Lipid oxidation occurs more readily in unsaturated fatty acid carbon chains, although SFA is also oxidized when exposed to heat during cooking (Mottram, 1998; Choe and Min, 2007). Therefore, the degree of saturation greatly influences the formation of lipid volatiles. As discussed previously, phospholipids in the polar lipid fraction are more unsaturated than the neutral lipid fraction (triglycerides); therefore, their impacts on lipid volatiles are much greater. Legako et al. (2015) reported that beef with greater USDA grade (more intramuscular fat or greater marbling) had more neutral lipids but not less phospholipids. Moreover, cooking changed PUFA and MUFA in the polar lipid fraction more than it did in the neutral lipid fraction. Mottram and Edwards (1983) also reported that meat flavor remained similar after neutral lipids were removed from muscle through solvent extraction. However, when both neutral and polar lipids were removed, cooked aromas changed dramatically. Elmore et al. (1999) changed n-3 PUFA in beef muscle by supplementing steers with bruised whole linseed (rich in α-linolenic; 18:3 n-3), eicosapentaenoic acid (20:5 n−3), fish oil (rich in eicosapentaenoic acid and docosahexaenoic acid, 22:6 n-3), or equal portions of linseed and fish oil. Steaks with greater PUFA produced more undesirable lipid oxidation products in cooked aromas—particularly n-alkanals, 2-alkenals, 1-alkanols, and alkylfurans—by up to 4-fold. These products are derived from the oxidation of MUFA and PUFA, promoted by adding more n-3 PUFA in the diet of the steers.
Thermal oxidation occurs through similar mechanisms and produces the same classes of compounds as autoxidation (Choe and Min, 2007). However, high temperature and optimal oxygen concentration during cooking drive volatile flavor compound development to the direction of more desirable volatile profiles. Under thermal oxidation, especially on the surface of the meat, unsaturated fatty acids are oxidized more quickly (Choe and Min, 2007; Song et al., 2011) and also polymerized to produce more oxygenated dimers and polymers (Nawar, 1984; Mottram, 1998). In addition, short-chain aldehydes, ketones, and alcohols are further oxidized to organic acids and esters (Song et al., 2011), lipid peroxides are polymerized to produce more oxygenated heterocyclic compounds such as cyclic carboxylic acids and their lactones (cyclic carboxylic esters), and SFA are also degraded to long-chain alkanes, aldehydes, and lactones (Nawar, 1984). This shift in volatile composition with less short-chain and unsaturated aldehydes and alcohols yields more desirable aromas, less volatility, and higher thresholds.
Although thermal oxidation of lipids occurs at as low as 60°C, the desirable composition of lipid-derived volatiles is produced at a temperature from 100°C up to 300°C (Wasserman, 1972) due to the rapid oxidation of unsaturated fatty acids and other oxidation products. The author documented lactones, alcohols, ketones, and short-chain fatty acids as the major volatiles produced at these high temperatures. Song et al. (2011) heated beef tallow at 140°C for 2 h with adequate airflow and documented a rapid increase in acidic value and a decrease in peroxide value with elevated temperature. Studies on thermal oxidation of lipids, such as the heating of frying oils, documented rapid hydrolysis of triglycerides into free fatty acids, especially when there is an abundance of water, and a high degree of oxidation of unsaturated fatty acids (Choe and Min, 2007). However, unlike autoxidation, many short-chain aldehydes and ketones are removed by the steam during cooking. They are also decomposed because of the high temperature or react with other compounds such as Maillard reaction products to produce more desirable volatiles.
The facts that short-chain aldehydes and ketones have higher aromatic thresholds than Maillard products (Mottram, 1998) and that they are decomposed and removed during cooking explain a much more desirable volatile composition of cooked meat compared with that of autoxidation. Reviews by both Choe and Min (2007) and Nawar (1984) document a high degree of dimerization and polymerization of unsaturated fatty acids, with dimeric compounds being predominant (Figure 9). The polymers can be radicalized to form cyclic compounds with carbonyl and hydroxyl groups (Nawar, 1984; Liu et al., 2020). Dimerization occurs through allyl radicals, hydroperoxides, intramolecular cyclization, and Diels-Alder reactions, in which conjugated PUFA react with other unsaturated linkages of other PUFA during cooking (Frankel et al., 1960, 1988; Frankel, 1980; Brühl, 2014). Among PUFA, long-chain PUFA—such as arachidonic acid—are also more susceptible to intermolecular cyclization (Figure 9). The intramolecular cyclization of long-chain PUFA and other C-C dimerization between unsaturated fatty acids form nonvolatile products, which further limits PUFA participation in the production of volatile compounds with offensive odors. However, these dimers and cyclic compounds are also susceptible to autoxidation in cooked meat and are sources of what is termed “hidden oxidation” because thermally dimerized compounds can be further autoxidized and decomposed to produce offensive volatile aromatic compounds during storage. Conversely, prolonged autoxidation may produce oxidative polymers, which decompose during cooking and yield off-odor volatiles (Frankel, 1980; Neff et al., 1988). Degradation of SFA and the production of lactone from hydroxy-fatty acids occur from thermal degradation during cooking. Several γ-lactones (Nawar, 1969; Song et al., 2011) come from γ-hydroxyalkanoic acids that are esterified with glycerol.
Several thermal oxidation products of fatty acids (ACD/ChemSketch, 2020; adapted from Nawar, 1984; Świzdor et al., 2012).
Saturated fatty acids are considered more stable than unsaturated ones; however, during cooking at 150°C or above—such as on the surface of grilled steaks—SFA are oxidized in a more complex pattern, yielding long-chain alkanes, alkanals, and ketones (Figure 9). If the oxygen attack occurs at C4 or C5 of the saturated carbon chain, γ- or δ-lactones will be formed. Song et al. (2011) reported an accumulation of hexanal, 1-octen-3-ol, (E,E)-2,4-decadienal, and (E,E)-2,4-heptadienal as contributors to the characteristics of beef flavor, whereas others have reported these volatile compounds as off-odors in meat products. The fact that only beef tallow was heated in this study implies the importance of flavor precursors from the lean portion and lipid–Maillard interactions that have been reviewed by Mottram (1998). The conditions for the Maillard reactions start early because proteins start to unfold at an internal temperature of 35°C and coagulate from 55°C to 80°C (Wasserman, 1972). Maillard browning, characterized by the brown color and polymerization of Maillard products, typically occur mildly in cooked meat unless meat is dehydrated at a high temperature. As the meat surface is roasted at 190°C, while fatty acids are extensively oxidized on the surface, milder Maillard reactions occur at an internal temperature of 60°C to 80°C (Wasserman, 1972), in which lipid-derived aldehydes are active participants, yielding some of the most characteristic volatiles of cooked meat aroma.
Interactions between lipid- and water-soluble flavor precursors
Under most cooking conditions, lipid flavor compounds are usually predominant. Fat also helps retain the aldehydes and ketones that are the most significant aroma contributors (Legako et al., 2016). It has traditionally been accepted that lipids are the source of characteristic flavors in meat (Hornstein and Crowe, 1960), through the formation of saturated and unsaturated aldehydes with 6 to 10 carbons. However, lipid volatile compounds have greater thresholds than volatiles derived from water-soluble compounds such as those participating in Maillard reactions (Mottram, 1998). Moreover, the interactions between lipid-derived compounds and Maillard compounds are probably more important for the development of meat flavor than originally thought. Legako et al. (2016) reported that USDA Standard steaks produced more n-aldehydes than USDA Prime and Low-Choice steaks. Although the percentage of PUFA in leaner meat is greater than that in fattier meat because of less total lipid content, Legako et al. (2015) reported that the PUFA content in USDA Standard steaks was still less than that in USDA Prime and Low-Choice steaks. Therefore, the development of lipid flavor compounds must also be influenced by the lean portion of meat, especially the water-soluble components, not just phospholipids. This interaction was researched in the early exploration of meat flavor chemistry. Wasserman and Spinelli (1970) extracted fat from pork tissues with chloroform and methanol and washed the extract with water. The original extract developed species-specific meat flavor after heating, whereas the water-washed portion retained only slightly cooked pork flavor, identified as a “piggy” note through 5,α-androst-16-en-3-one, the boar odor dissolved in pork fat. Pippen and Mecchi (1969) demonstrated that lipid from chicken adipose tissue had no chicken aroma after removing polar compounds by water washing. The authors also reported that hydrogen sulfide reacted with acetaldehyde to form an odorous compound and suggested that flavor compounds were formed interactively between lipid and water-soluble precursors. Sanderson et al. (1966) also reported that heating beef fat together with beef lean yielded much more flavor carbonyls than heating beef fat alone. Myers et al. (2009) reported that beef and pork lean was more important for species-specific flavor, although fat level affected flavor intensity. As discussed previously, the lamb-specific flavor results not only from branched-chain fatty acids that are not found in other species (Mottram, 1998) but also from the reactions between lipid oxidation products and Maillard products, forming various alkyl-substituted flavor compounds such as alkylthiazoles, alkylpyrazines, and alkylpyridines. Lipid oxidation products participating in Maillard reactions are most likely aldehydes that compete with carbonyls from reducing sugars for amino compounds (Zamora and Hidalgo, 2011). Compounds such as alkyldimethylpyrazines and alkyltrithiolanes (Mottram, 1998) are formed when lipid-derived aldehydes enter Maillard reactions with either pyrazines or hydrogen sulfide, respectively (Figure 10). Compounds such as 2-pentylpyridine (Figure 10) are produced from the reactions between lipid aldehydes and ammonia, a byproduct of Maillard reactions. Recently, this competition was shown to produce various characteristic and desirable flavor compounds in cooked meat (Zamora and Hidalgo, 2011; Kosowska et al., 2017). Myer et al. (2009) reported an increase in flavor intensity as the authors increased the internal temperature of beef and pork patties from 66°C to 71°C. These authors also suggested that flavor precursors in the lean portion of meats are more important to the species-specific aromas, which substantiates the previous discussion in this manuscript. These authors suggested that lower temperatures such as 66°C did not allow Maillard reactions to occur, thereby possibly hindering the lipid–Maillard pathways as well. Gardner and Legako (2018) found that dimethyl- and trimethylpyrazine concentrations in the headspace of ground beef were greater at higher temperatures. Their data indicated that such an increase was more drastic in USDA Prime beef than in USDA Choice and Standard. USDA Prime beef has more fat, and these pyrazines are products of lipid–Maillard interactions. These findings imply that fatty acid oxidation products are not simply flavor compounds; they are also precursors for complex interactions with Maillard products to form a more characteristic and desirable cooked meat flavor. Such interactions do not occur in autoxidation during the storage of meats.
Several volatile compounds from lipid–Maillard interactions (ACD/ChemSketch, 2020; adapted from Mottram, 1998).
Off-flavor compounds from lipid oxidation
During refrigerated storage at 1°C to 7°C, lipid oxidation occurs slowly with minimal interactions with other compounds such as Maillard products and yields various off-odor compounds that are detrimental to meat quality. The heat used in cooking not only accelerates lipid oxidation and produces a desirable profile of lipid carbonyls and carboxylic acids for cooked meat flavor but also facilitates the hydrolysis of fatty acids from triglycerides or phospholipids. This hydrolysis allows fatty acids to be more reactive and produce more carbonyls more quickly (Wasserman, 1972). Although data from the early literature indicated that lower temperature (71°C) produced a less intense beef flavor than higher temperature (77°C; Berry, 1994; Kregel et al., 1986), Myer et al. (2009) disagreed because their patties cooked to 71°C internal temperature exhibited intense characteristic meat flavor. However, these authors also documented a less intense meat flavor at 66°C internal temperature. They suggested that lower temperatures such as 66°C did not allow Maillard reactions to occur. As discussed previously, if Maillard reactions occur to a minimal degree, lipid oxidation products—without much interaction with Maillard products—tend to produce a less desirable volatile composition.
At lower temperatures in storage conditions, autoxidation of meat produces short-chain aldehydes and alcohols that have offensive odors (Ismail et al., 2008). Although aldehydes from the Maillard reactions such as 2,3-butanedione (Hunt et al., 2016) are desirable, major aldehydes from the autoxidation of meat lipids such as hexanal are undesirable. Ross and Smith (2006) reviewed various studies on lipid oxidation in muscle foods and selected aldehydes such as octanal, heptanal, pentanal, and hexanal as the best indicators of lipid autoxidation. Yancey et al. (2006) also reported that 2-decenal-[E]-, 2-undecenal, and propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester in beef were indicative of a strong livery odor. Another phenomenon called “warmed-over,” coined first by Younathan and Watts (1959), describes the rancidity onset of cooked meat after a refrigeration period. The warmed-over flavor is caused by the continuous oxidation of lipids after cooking and can be estimated by measuring the thiobarbituric acid reactive substances (Wilson et al., 1976). This value primarily measures malondialdehyde, a secondary oxidation product from linolenic acid (Frankel, 1980), although this assay has been shown to include various aldehydes and other interferences from proteins and PUFA (Draper et al., 1993; Jardine et al., 2002). Angelo et al. (1987) reported that warmed-over flavor was characterized by hexanal and 2,3-octanedione, both of which are products of lipid oxidation. Studies in beef and pork have consistently identified these 2 compounds as warmed-over flavor markers (Kerler and Grosch, 1996; Akcan et al., 2017). However, as discussed previously, other lipid thermal oxidation products such as dimers and cyclic compounds are also oxidized and decomposed to offensive aromas during the storage of cooked meat.
Conclusions
Meat from various species has similar fatty acids, with palmitic, stearic, oleic, palmitoleic, linoleic, linolenic, and arachidonic acids being the most predominant; however, their concentrations and percentages vary greatly. Autoxidation of unsaturated fatty acids causes off-odors, whereas the lipid-derived volatile profile is more desirable under thermal oxidation and in the reactions with other flavor compounds such as Maillard reaction products. Recent research has suggested that the development of lipid flavor compounds is influenced by the lean portion of meat. Therefore, the interactions among lipid-derived water-soluble, and Maillard compounds are probably more important than originally thought and warrant further research.
Literature Cited
Aberle, E. D., J. C. Forrest, D. E. Gerrard, and E. W. Mills. 2001. Principles of meat science. Kendall Hunt Publishing, Dubuque, IA.
ACD/ChemSketch. 2020. Version 2017.2.1. Advanced Chemistry Development, Inc., Toronto, ON, Canada.
Akcan, T., M. Estévez, S. Rico, S. Ventanas, and D. Morcuende. 2017. Hawberry (Crataegus monogyna Jaqc.) extracts inhibit lipid oxidation and improve consumer liking of ready-to-eat (RTE) pork patties. J. Food Sci. Tech. Mys. 54:1248–1255. doi: https://doi.org/10.1007/s13197-017-2578-8.
Amaral, A. B., M. V. D. Silva, and S. C. D. S. Lannes. 2018. Lipid oxidation in meat: Mechanisms and protective factors–a review. Food Sci. Tech.-Brazil. 38:1–15. doi: https://doi.org/10.1590/fst.32518.
Angelo, A. S., J. R. Vercellotti, M. G. Legendre, C. H. VinnelT, J. W. Kuan, C. James Jr., and H. P. Dupuy. 1987. Chemical and instrumental analyses of warmed-over flavor in beef. J. Food Sci. 52:1163–1168. doi: https://doi.org/10.1111/j.1365-2621.1987.tb14034.x.
Arshad, M. S., M. Sohaib, R. S. Ahmad, M. T. Nadeem, A. Imran, M. U. Arshad, J. H. Kwon, and Z. Amjad. 2018. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 17:223. doi: https://doi.org/10.1186/s12944-018-0860-z.
Banskalieva, V., T. A. Sahlu, and A. L. Goetsch. 2000. Fatty acid composition of goat muscles and fat depots: A review. Small Ruminant Res. 37:255–268. doi: https://doi.org/10.1016/S0921-4488(00)00128-0.
Belaunzaran, X., P. Lavín, L. J. Barron, A. R. Mantecón, J. K. Kramer, and N. Aldai. 2017. An assessment of the fatty acid composition of horse-meat available at the retail level in northern Spain. Meat Sci. 124:39–47. doi: https://doi.org/10.1016/j.meatsci.2016.10.014.
Berry, B. W. 1994. Fat level, high temperature cooking and degree of doneness affect sensory, chemical and physical properties of beef patties. J. Food Sci. 59:10–14. doi: https://doi.org/10.1111/j.1365-2621.1994.tb06885.x.
Bessa, R. J., S. P. Alves, and J. Santos-Silva. 2015. Constraints and potentials for the nutritional modulation of the fatty acid composition of ruminant meat. Eur. J. Lipid Sci. Tech. 117:1325–1344. doi: https://doi.org/10.1002/ejlt.201400468.
Brassard, M. E., P. Y. Chouinard, R. Gervais, É. Pouliot, C. Gariépy, and D. Cinq-Mars. 2017. Effects of level of barley and corn in concentrate-fed Boer kids on growth performance, meat quality, and muscle fatty acid composition. Can. J. Anim. Sci. 98:156–165. doi: https://doi.org/10.1139/cjas-2017-0026.
Bravo-Lamas, L., L. J. Barron, L. Farmer, and N. Aldai. 2018. Fatty acid composition of intramuscular fat and odour-active compounds of lamb commercialized in northern Spain. Meat Sci. 139:231–238. doi: https://doi.org/10.1016/j.meatsci.2018.02.006.
Bravo-Lamas, L., L. J. Barron, J. K. Kramer, I. Etaio, and N. Aldai. 2016. Characterization of the fatty acid composition of lamb commercially available in northern Spain: Emphasis on the trans-18: 1 and CLA content and profile. Meat Sci. 117:108–116. doi: https://doi.org/10.1016/j.meatsci.2016.02.043.
Brennand, C. P., and R. C. Lindsay. 1992. Distribution of volatile branched-chain fatty acids in various lamb tissues. Meat Sci. 31:411–421. doi: https://doi.org/10.1016/0309-1740(92)90024-X.
Brockerhoff, H., R. J. Hoyle, and N. Wolmark. 1966. Positional distribution of fatty acids in triglycerides of animal depot fats. BBA-Lipid Lipid Met. 116:67–72. doi: https://doi.org/10.1016/0005-2760(66)90092-0.
Brühl, L. 2014. Fatty acid alterations in oils and fats during heating and frying. Eur. J. Lipid Sci. Tech. 116:707–715. doi: https://doi.org/10.1002/ejlt.201300273.
Brzozowska, A. M., and J. Oprządek. 2016. Metabolism of fatty acids in tissues and organs of the ruminants - A review. Anim. Sci. Pap. Rep. 34:211–220.
Buttery, R. G., L. C. Ling, R. Teranishi, and T. R. Mon. 1977. Roasted lamb fat: Basic volatile components. J. Agr. Food Chem. 25:1227–1229. doi: https://doi.org/10.1021/jf60214a038.
Calkins, C. R., and J. M. Hodgen. 2007. A fresh look at meat flavor. Meat Sci. 77:63–80. doi: https://doi.org/10.1016/j.meatsci.2007.04.016.
Camareno, K. C., A. T. Sukumaran, J. Scott, N. Gurung, T. T. N. Dinh, and D. D. Burnett. 2016. Effects of feeding varying levels of deoiled distillers dried grains with solubles on fatty acid composition of subcutaneous adipose tissue in meat goats. J. Anim. Sci. 94(suppl_5):825–825. doi: https://doi.org/10.2527/jam2016-1693.
Choe, E., and D. B. Min. 2007. Chemistry of deep-fat frying oils. J. Food Sci. 72:R77–R86. doi: https://doi.org/10.1111/j.1750-3841.2007.00352.x.
Daley, C. A., A. Abbott, P. S. Doyle, G. A. Nader, and S. Larson. 2010. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 9:10. doi: https://doi.org/10.1186/1475-2891-9-10.
De Smet, S., K. Raes, and D. Demeyer. 2004. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 53:81–98. doi: https://doi.org/10.1051/animres:2004003.
de Tonnac, A., M. Guillevic, and J. Mourot. 2018. Fatty acid composition of several muscles and adipose tissues of pigs fed n-3 PUFA rich diets. Meat Sci. 140:1–8. doi: https://doi.org/10.1016/j.meatsci.2017.11.023.
Dinh, T., and L. Thompson. 2016. Cholesterol: Properties, processing effects, and determination. Encyclopedia of Food and Health. 60–69. doi: https://doi.org/10.1016/B978-0-12-384947-2.00150-1.
Dinh, T. T. N., J. R. Blanton Jr., D. G. Riley, C. C. Chase Jr., S. W. Coleman, W. A. Phillips, J. C. Brooks, M. F. Miller, and L. D. Thompson. 2010. Intramuscular fat and fatty acid composition of longissimus muscle from divergent pure breeds of cattle. J. Anim. Sci. 88:756–766. doi: https://doi.org/10.2527/jas.2009-1951.
Dinh, T. T. N., J. F. Legako, M. F. Miller, and J. C. Brooks. 2018. Effects of USDA quality grade and cooking on water-soluble precursors of beef flavor. Meat Sci. 146:122–130. doi: https://doi.org/10.1016/j.meatsci.2018.08.008.
Domínguez, R., M. Pateiro, M. Gagaoua, F. J. Barba, W. Zhang, and J. M. Lorenzo. 2019. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 8:429. doi: https://doi.org/10.3390/antiox8100429.
Draper, H. H., E. J. Squires, H. Mahmoodi, J. Wu, S. Agarwal, and M. Hadley. 1993. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radical Bio. Med. 15:353–363. doi: https://doi.org/10.1016/0891-5849(93)90035-S.
Duncan, S. E. 2001. Lipids: Basic concepts. In: G. L. Christen and J. S. Smith, editors, Food chemistry: Principles and application. Science Technology System, CA.
Elmore, J. S., D. S. Mottram, M. Enser, and J. D. Wood. 1999. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agr. Food Chem. 47:1619–1625. doi: https://doi.org/10.1021/jf980718m.
Elmore, J. S., H. E. Warren, D. S. Mottram, N. D. Scollan, M. Enser, R. I. Richardson, and J. D. Wood. 2004. A comparison of the aroma volatiles and fatty acid compositions of grilled beef muscle from Aberdeen Angus and Holstein-Friesian steers fed diets based on silage or concentrates. Meat Sci. 68:27–33. doi: https://doi.org/10.1016/j.meatsci.2004.01.010.
Faustman, C., Q. Sun, R. Mancini, and S. P. Suman. 2010. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 86:86–94. doi: https://doi.org/10.1016/j.meatsci.2010.04.025.
Ferjak, E. N., C. A. Cavinder, A. T. Sukumaran, D. D. Burnett, C. O. Lemley, and T. T. N. Dinh. 2019. Fatty acid composition of mesenteric, cardiac, abdominal, intermuscular, and subcutaneous adipose tissues from horses of three body condition scores. Livest. Sci. 223:116–123. doi: https://doi.org/10.1016/j.livsci.2019.02.010.
Frank, D., K. Kaczmarska, J. Paterson, U. Piyasiri, and R. Warner. 2017. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat Sci. 133:61–68. doi: https://doi.org/10.1016/j.meatsci.2017.06.006.
Frankel, E. N. 1980. Lipid oxidation. Prog. Lipid Res. 19:1–22. doi: https://doi.org/10.1016/0163-7827(80)90006-5.
Frankel, E. N. 1984. Lipid oxidation: Mechanisms, products and biological significance. J. Am. Oil Chem. Soc. 61:1908–1917. doi: https://doi.org/10.1007/BF02540830.
Frankel, E. N. 1991. Recent advances in lipid oxidation. J. Sci. Food Agr. 54:495–511. doi: https://doi.org/10.1002/jsfa.2740540402.
Frankel, E. N., C. D. Evans, and J. C. Cowan. 1960. Thermal dimerization of fatty ester hypdroperoxides. J. Am. Oil Chem. Soc. doi: https://doi.org/10.1007/BF02631198.
Frankel, E. N., W. E. Neff, E. Selke, and D. D. Brooks. 1988. Analysis of autoxidized fats by gas chromatography-mass spectrometry: X. Volatile thermal decomposition products of methyl linolenate dimers. Lipids. 23:295–298. doi: https://doi.org/10.1007/BF02537336.
French, P., C. Stanton, F. Lawless, E. G. O’Riordan, F. J. Monahan, P. J. Caffrey, and A. P. Moloney. 2000. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. J. Anim. Sci. 78:2849–2855. doi: https://doi.org/10.2527/2000.78112849x.
Fruet, A. P. B., F. Trombetta, F. S. Stefanello, C. S. Speroni, J. Z. Donadel, A. N. M. De Souza, A. Rosado Júnior, C. J. Tonetto, R. Wagner, A. De Mello, and J. L. Nörnberg. 2018. Effects of feeding legume-grass pasture and different concentrate levels on fatty acid profile, volatile compounds, and off-flavor of the M. longissimus thoracis. Meat Sci. 140:112–118. doi: https://doi.org/10.1016/j.meatsci.2018.03.008.
Gardner, K., and J. F. Legako. 2018. Volatile flavor compounds vary by beef product type and degree of doneness. J. Anim. Sci. 96:4238–4250. doi: https://doi.org/10.1093/jas/sky287.
Grosch, W. 1982. Lipid degradation products and flavours. In: I. D. Morton and A. J. MacLeod, editors, Food flavours. Elsevier, Amsterdam. pp. 325–398. doi: https://doi.org/10.1007/978-1-4615-2167-9_7.
Gunstone, F. D. 1996. Fatty acids—Nomenclature, structure, isolation and structure determination, biosynthesis and chemical synthesis. In: Fatty acid and lipid chemistry, Springer, Boston, MA. p. 1–34.
Gunstone, F. D. 2012. Fatty acid and lipid chemistry. Springer, Boston, MA.
Hornstein, I., and P. F. Crowe. 1960. Meat flavor chemistry, flavor studies on beef and pork. J. Agr. Food Chem. 8:494–498. doi: https://doi.org/10.1021/jf60112a022.
Hunt, M. R., J. F. Legako, T. T. N. Dinh, A. J. Garmyn, T. G. O’Quinn, C. H. Corbin, R. J. Rathmann, J. C. Brooks, and M. F. Miller. 2016. Assessment of volatile compounds, neutral and polar lipid fatty acids of four beef muscles from USDA Choice and Select graded carcasses and their relationships with consumer palatability scores and intramuscular fat content. Meat Sci. 116:91–101. doi: https://doi.org/10.1016/j.meatsci.2016.02.010.
Ismail, H. A., E. J. Lee, K. Y. Ko, and D. U. Ahn. 2008. Effects of aging time and natural antioxidants on the color, lipid oxidation and volatiles of irradiated ground beef. Meat Sci. 80:582–591. doi: https://doi.org/10.1016/j.meatsci.2008.02.007.
Jardine, D., M. Antolovich, P. D. Prenzler, and K. Robards. 2002. Liquid chromatography–mass spectrometry (LC-MS) investigation of the thiobarbituric acid reactive substances (TBARS) reaction. J. Agr. Food Chem. 50:1720–1724. doi: https://doi.org/10.1021/jf011336a.
Jenkins, T. C. 1993. Lipid metabolism in the rumen. J. Dairy Sci. 76:3851–3863. doi: https://doi.org/10.3168/jds.S0022-0302(93)77727-9.
Jiang, J., X. Tang, Y. Xue, G. Lin, and Y. L. Xiong. 2017. Dietary linseed oil supplemented with organic selenium improved the fatty acid nutritional profile, muscular selenium deposition, water retention, and tenderness of fresh pork. Meat Sci. 131:99–106. doi: https://doi.org/10.1016/j.meatsci.2017.03.014.
Kerler, J., and W. Grosch. 1996. Odorants contributing to warmed-over flavor (WOF) of refrigerated cooked beef. J. Food Sci. 61:1271–1275. doi: https://doi.org/10.1111/j.1365-2621.1996.tb10977.x.
Khan, N. A., J. W. Cone, V. Fievez, and W. H. Hendriks. 2012. Causes of variation in fatty acid content and composition in grass and maize silages. Anim. Feed Sci. Tech. 174:36–45. doi: https://doi.org/10.1016/j.anifeedsci.2012.02.006.
Khan, M. I., C. Jo, and M. R. Tariq. 2015. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 110:278–284. doi: https://doi.org/10.1016/j.meatsci.2015.08.002.
King, M. F., B. L. Hamilton, M. A. Matthews, D. C. Rule, and R. A. Field. 1993. Isolation and identification of volatiles and condensable material in raw beef with supercritical carbon dioxide extraction. J. Agr. Food Chem. 41:1974–1981. doi: https://doi.org/10.1021/jf00035a030.
Klopfenstein, T. J., G. E. Erickson, and V. R. Bremer. 2008. BOARD-INVITED REVIEW: Use of distillers by-products in the beef cattle feeding industry. J. Anim. Sci. 86:1223–1231. doi: https://doi.org/10.2527/jas.2007-0550.
Komprda, T., M. Jůzl, M. Matejovičová, L. Levá, M. Piechowiczová, Š. Nedomová, V. Popelková, and P. Vymazalová. 2020. Effect of high dietary level (8%) of fish oil on long-chain polyunsaturated fatty acid n-3 content in pig tissues and plasma biochemical parameters. Animals. 10:1657. doi: https://doi.org/10.3390/ani10091657.
Kosowska, M., M. A. Majcher, and T. Fortuna. 2017. Volatile compounds in meat and meat products. Food Sci. Tech.-Brazil. 37:1–7. doi: https://doi.org/10.1590/1678-457X.08416.
Kouba, M., and J. Mourot. 2011. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie. doi: https://doi.org/10.1016/j.biochi.2010.02.027.
Kregel, K. K., K. J. Prusa, and K. V. Hughes. 1986. Cholesterol content and sensory analysis of ground beef as influenced by fat level, heating, and storage. J. Food Sci. 51:1162–1165. doi: https://doi.org/10.1111/j.1365-2621.1986.tb13073.x.
Legako, J. F., T. T. N. Dinh, M. F. Miller, K. Adhikari, and J. C. Brooks. 2016. Consumer palatability scores, sensory descriptive attributes, and volatile compounds of grilled beef steaks from three USDA Quality Grades. Meat Sci. 112:77–85. doi: https://doi.org/10.1016/j.meatsci.2015.10.018.
Legako, J. F., T. T. N. Dinh, M. F. Miller, and J. C. Brooks. 2015. Effects of USDA beef quality grade and cooking on fatty acid composition of neutral and polar lipid fractions. Meat Sci. 100:246–255. doi: https://doi.org/10.1016/j.meatsci.2014.10.013.
Leheska, J. M., L. D. Thompson, J. C. Howe, E. Hentges, J. Boyce, J. C. Brooks, B. Shriver, L. Hoover, and M. F. Miller. 2008. Effects of conventional and grass-feeding systems on the nutrient composition of beef. J. Anim. Sci. 86:3575–3585. doi: https://doi.org/10.2527/jas.2007-0565.
Liu, H., Y. Chen, C. Shi, X. Yang, and D. Han. 2020. FT-IR and Raman spectroscopy data fusion with chemometrics for simultaneous determination of chemical quality indices of edible oils during thermal oxidation. LWT-Food Sci. Technol. 119:108906. doi: https://doi.org/10.1016/j.lwt.2019.108906.
Lobb, K., and C. K. Chow. 2000. Fatty acid classification and nomenclature. In: C. K. Chow, editor, Fatty acids in foods and their health implications. CRC Press, Boca Raton, FL. pp. 1–16.
Madruga, M., I. Dantas, A. Queiroz, L. Brasil, and Y. Ishihara. 2013. Volatiles and water-and fat-soluble precursors of Saanen goat and cross Suffolk lamb flavour. Molecules. 18:2150–2165. doi: https://doi.org/10.3390/molecules18022150.
Mottram, D. S. 1998. Flavour formation in meat and meat products: A review. Food Chem. 62:415–424. doi: https://doi.org/10.1016/S0308-8146(98)00076-4.
Mottram, D. S., and R. A. Edwards. 1983. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agr. 34:517–522.
Myers, A. J., S. M. Scramlin, A. C. Dilger, C. M. Souza, F. K. McKeith, and J. Killefer. 2009. Contribution of lean, fat, muscle color and degree of doneness to pork and beef species flavor. Meat Sci. 82:59–63. doi: https://doi.org/10.1002/jsfa.2740340513.
Nawar, W. W. 1969. Thermal degradation of lipids. A review. J. Agr. Food Chem. 17:18–21. doi: https://doi.org/10.1021/jf60161a012.
Nawar, W. W. 1984. Chemical changes in lipids produced by thermal processing. J. Chem. Educ. 61:299. doi: https://doi.org/10.1021/ed061p299.
Neff, W. E., E. N. Frankel, and K. Fujimoto. 1988. Autoxidative dimerization of methyl linolenate and its monohydroperoxides, hydroperoxy epidioxides and dihydroperoxides. J. Am. Oil Chem. Soc. 65:616–623. doi: https://doi.org/10.1007/BF02540690.
Nuernberg, K., D. Dannenberger, G. Nuernberg, K. Ender, J. Voigt, N. D. Scollan, J. D. Wood, G. R. Nute, and R. I. Richardson. 2005. Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livest. Prod. Sci. 94:137–147. doi: https://doi.org/10.1016/j.livprodsci.2004.11.036.
Oliveira, M. A., S. P. Alves, J. Santos-Silva, and R. J. Bessa. 2017. Effect of dietary starch level and its rumen degradability on lamb meat fatty acid composition. Meat Sci. 123:166–172. doi: https://doi.org/10.1016/j.meatsci.2016.10.001.
Palmquist, D. L., and T. C. Jenkins. 1980. Fat in lactation rations. J. Dairy Sci. 63:1–14. doi: https://doi.org/10.3168/jds.S0022-0302(80)82881-5.
Pegg, R. B., and F. Shahidi. 2012. Off flavors and rancidity in foods. In: L. M. L. Nollet, editor, Handbook of meat, poultry and seafood quality. p. 127–139. doi: https://doi.org/10.1002/9781118352434.
Pippen, E. L., and E. P. Mecchi. 1969. Hydrogen sulfide, a direct and potentially indirect contributor to cooked chicken aroma. J. Food Sci. 34:443–446. doi: https://doi.org/10.1111/j.1365-2621.1969.tb12800.x.
Rhee, K. S., D. F. Waldron, Y. A. Ziprin, and K. C. Rhee. 2000. Fatty acid composition of goat diets vs intramuscular fat. Meat Sci. 54:313–318. doi: https://doi.org/10.1016/s0309-1740(99)00094-7.
Ribeiro, C. V. D. M., D. E. Oliveira, S. O. Juchem, T. M. Silva, and E. S. Nalério. 2011. Fatty acid profile of meat and milk from small ruminants: A review. Rev. Bras. Zootecn. 40:S121–S137.
Ross, C. F., and D. M. Smith. 2006. Use of volatiles as indicators of lipid oxidation in muscle foods. Compr. Rev. Food Sci. F. 5:18–25. doi: https://doi.org/10.1111/j.1541-4337.2006.tb00077.x.
Sanderson, A., A. M. Pearson, and B. S. Schweigert. 1966. Effect of cooking procedure on flavor components of beef. Carbonyl compounds. J. Agr. Food Chem. 14:245–247. doi: https://doi.org/10.1021/jf60145a013.
Schingoethe, D. J., K. F. Kalscheur, A. R. Hippen, and A. D. Garcia. 2009. The use of distillers products in dairy cattle diets. J. Dairy Sci. 92:P5802–5813. doi: https://doi.org/10.3168/jds.2009-2549.
Schmid, A., M. Collomb, R. Sieber, and G. Bee. 2006. Conjugated linoleic acid in meat and meat products: A review. Meat Sci. 73:29–41. doi: https://doi.org/10.1016/j.meatsci.2005.10.010.
Sébédio, J. L., and W. M. Ratnayake. 2008. Analysis of trans mono-and polyunsaturated fatty acids. In: A. J. Dijkstra, R. J. Hamilton, and W. Hamm, editors, Trans fatty acids. Blackwell Publishing, Hoboken, NJ. p. 102–131.
Shingfield, K. J., M. Bonnet, and N. D. Scollan. 2013. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal. 7(Suppl. 1):132–162. doi: https://doi.org/10.1017/S1751731112001681.
Song, S., X. Zhang, K. Hayat, P. Liu, C. Jia, S. Xia, Z. Xiao, H. Tian, and Y. Niu. 2011. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 124:203–209. doi: https://doi.org/10.1016/j.foodchem.2010.06.010.
Świzdor, A., A. Panek, N. Milecka-Tronina, and T. Kołek. 2012. Biotransformations utilizing β-oxidation cycle reactions in the synthesis of natural compounds and medicines. Int. J. Mol. Sci. 13:16514–16543. doi: https://doi.org/10.3390/ijms131216514.
Tonial, I., A. C. Aguia, C. C. Oliveira, E. G. Bonnafé, J. V. Visentainer, and N. E. De Souza. 2009. Fatty acid and cholesterol content, chemical composition and sensory evaluation of horsemeat. S. Afr. J. Anim. Sci. 39(4). doi: https://doi.org/10.4314/sajas.v39i4.51135.
USDA. 2021. FoodData Central. U.S. Department of Agriculture Agricultural Research Service. fdc.nal.usda.gov. (Accessed 8 April 2021).
Um, K. W., M. E. Bailey, A. D. Clarke, and R. R. Chao. 1992. Concentration and identification of volatile compounds from heated beef fat using supercritical carbon dioxide extraction-gas liquid chromatography/mass spectrometry. J. Agr. Food Chem. 40:1641–1646. doi: https://doi.org/10.1021/JF00021A033.
Voet, D., J. G. Voet, and C. W. Pratt. 2006. Fundamentals of biochemistry: Life at the molecular level. Wiley, Hoboken, NJ. p. 1264.
Wahle, K. W., S. D. Heys, and D. Rotondo. 2004. Conjugated linoleic acids: Are they beneficial or detrimental to health? Prog. Lipid Res. 43:553–587. doi: https://doi.org/10.1016/j.plipres.2004.08.002.
Wallis, J. G., J. L. Watts, and J. Browse. 2002. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 27:467–473. doi: https://doi.org/10.1016/s0968-0004(02)02168-0.
Wang, Y., H. Song, Y. Zhang, J. Tang, and D. Yu. 2016. Determination of aroma compounds in pork broth produced by different processing methods. Flavour Frag. J. 31:319–328. doi: https://doi.org/10.1002/ffj.3320.
Wang, X., Y. Xie, X. Li, Y. Liu, and W. Yan. 2018. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 146:154–159. doi: https://doi.org/10.1016/j.meatsci.2018.08.011.
Warren, H. E., N. D. Scollan, M. Enser, S. I. Hughes, R. I. Richardson, and J. D. Wood. 2008. Effects of breed and a concentrate or grass silage diet on beef quality in cattle of 3 ages. I: Animal performance, carcass quality and muscle fatty acid composition. Meat Sci. 78:256–269. doi: https://doi.org/10.1016/j.meatsci.2007.06.008.
Wasserman, A. E. 1972. Thermally produced flavor components in the aroma of meat and poultry. J. Agr. Food Chem. 20:737–741.
Wasserman, A. E., and A. M. Spinelli. 1970. Sugar-amino acid interaction in the diffusate of water extract of beef and model systems. J. Food Sci. 35:328–332. doi: https://doi.org/10.1111/j.1365-2621.1970.tb12177.x.
Wilson, B. R., A. M. Pearson, and F. B. Shorland. 1976. Effect of total lipids and phospholipids on warmed-over flavor in red and white muscle from several species as measured by thiobarbituric acid analysis. J. Agr. Food Chem. 24:7–11. doi: https://doi.org/10.3382/ps.0660458.
Wood, J. D., M. Enser, A. V. Fisher, G. R. Nute, P. R. Sheard, R. I. Richardson, S. I. Hughes, and F. M. Whittington. 2008. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 78:343–358. doi: https://doi.org/10.1016/j.meatsci.2007.07.019.
Yancey, E. J., J. P. Grobbel, M. E. Dikeman, J. S. Smith, K. A. Hachmeister, E. C. Chambers IV, P. Gadgil, G. A. Milliken, and E. A. Dressler. 2006. Effects of total iron, myoglobin, hemoglobin, and lipid oxidation of uncooked muscles on livery flavor development and volatiles of cooked beef steaks. Meat Sci. 73:680–686. doi: https://doi.org/10.1016/j.meatsci.2006.03.013.
Younathan, M. T., and B. M. Watts. 1959. Relationship of meat pigments to lipid oxidation. J. Food Sci. 6:728–734. doi: https://doi.org/10.1111/j.1365-2621.1959.tb17326.x.
Yu, K., and G. Shu. 2013. Fatty acid and transcriptome profiling of longissimus dorsi muscles between pig breeds differing in meat quality. Int. J. Biol. Sci. 9:108. doi: https://doi.org/10.7150/ijbs.5306.
Zamora, R., and F. J. Hidalgo. 2011. The Maillard reaction and lipid oxidation. Lipid Technology. 23:59–62. doi: https://doi.org/10.1002/lite.201100094.
Zhao, J., T. Wang, J. Xie, Q. Xiao, J. Cheng, F. Chen, S. Wang, and B. Sun. 2019. Formation mechanism of aroma compounds in a glutathione-glucose reaction with fat or oxidized fat. Food Chem. 270:436–444. doi: https://doi.org/10.1016/j.foodchem.2018.07.106.