Introduction
Consumers routinely select or reject meat products based on color and appearance, so suppliers of muscle food products must create and maintain the desired color attributes. Color of muscle foods revolves around myoglobin, the primary red pigment in meat. However, ultimate perceived color is affected by many factors, such as species, animal genetics and nutritional background, postmortem changes in muscle (especially the dynamics of pH and meat temperature decline), inter- and intramuscular effects, postmortem storage temperatures and time, and a whole host of processing (including antimicrobial interventions), packaging, and display and lighting variables.
Color evaluation is an essential part of meat research, product development, and troubleshooting of processing problems. When done properly, both visual and instrumental appraisals of color are powerful and useful research tools for meat scientists. However, these evaluations must be conducted using carefully designed procedures to avoid artifacts or biased data. Simply put, complete color evaluations usually cannot be done with only one scale, sampling technique, or instrumental measurement. Measurements of color and color stability are complex and often misused in routine work.
Details of measurement must be reported for the proper interpretation of data. Tapp et al. (2011) surveyed 1,068 articles and found that 73.6% of researchers failed to report aperture size, 52.4% number of scans per sample, 48.9% illuminant used, 65.7% angle of observation, and nearly 3% failed to report the type of instrument used. Up to 8.4% did not indicate the method used to calculate tristimulus values (such as Commission Internationale de l’Eclairage [CIE] L*a*b* vs. Hunter Lab), nor did they state whether universally accepted revisions of the 1976 CIE for the calculation of CIE L*a*b* (CIE, 1976) were followed. Only 24.8% of the articles calculated hue angle, and only 25.5% calculated saturation index. Similar inconsistencies likely also exist for the reporting of visual color evaluations. These guidelines should encourage more uniform reporting of pertinent experimental details and sample properties for studies involving visual and/or instrumental color evaluation.
Moreover, these guidelines provide suggestions for researchers investigating the biological basis of meat color. A thorough review of the entirety of these guidelines is strongly suggested to those new to meat color research. However, users should be able to pick and choose the background information needed to ensure that their efforts result in reliable and accurate appraisals of color. These guidelines are intended for use in planning and executing investigations involving meat color. Investigators must integrate the principles detailed in these guidelines into their experimental design to address the specific question of interest.
Myoglobin Chemistry
Fundamental myoglobin chemistry
Myoglobin is the water-soluble protein responsible for meat color. Within the 8 α-helices (often labeled A–H) of myoglobin, a prosthetic heme group containing a centrally located iron atom is positioned in the protein’s hydrophobic core. Of the 6 bonds associated with this iron atom, 4 connect iron to the heme ring, the 5th attaches to the proximal histidine-93, and the 6th site is available to reversibly bind ligands including diatomic oxygen, carbon monoxide (CO), water, and nitric oxide (NO). The ligand present at the 6th coordination site and the valence state of iron determine meat color via 4 chemical forms of myoglobin: deoxymyoglobin (DMb), oxymyoglobin (OMb), carboxymyoglobin (COMb), and metmyoglobin (MMb) (see Figure 1).
Deoxymyoglobin results in a dark purplish-red or purplish-pink color typical of the interior color of fresh meat and that in vacuum packages. Deoxymyoglobin contains ferrous iron (Fe2+) with a vacant (no ligand attached) 6th coordination site. To maintain DMb, very low oxygen tension (< 1.4 mm Hg) within vacuum packages or the interior of muscle is necessary. Oxygenation of DMb forms a bright red color via the formation of OMb, which has diatomic oxygen attached to the 6th coordination site of Fe2+. The oxygen ligand also interacts with the distal histidine-64, producing a more compact protein structure than DMb, which has no ligand present to link iron to the distal histidine. Carboxymyoglobin formation occurs when CO attaches to the vacant 6th position of DMb, producing a stable bright red color when the environment is devoid of oxygen. Atmospheres containing oxygen (albeit concentration dependent) will result in the conversion of COMb to either OMb or MMb. Metmyoglobin is the oxidized form of myoglobin with a ferric iron (Fe3+), resulting in a tan- to brown-colored form of myoglobin. Typically, MMb forms easily at 1% to 3% oxygen, which is equivalent to oxygen partial pressures of 1 to 25 torr (see “Partial Pressure of Gases in Meat Packages” in Appendix E). Water is the ligand at the 6th position of the iron in MMb.
Dynamics of myoglobin redox form interconversions
Myoglobin oxygenation or blooming (reaction 1 in Figure 1) depends on time, temperature, pH, and competition for oxygen by mitochondria. More specifically, the competition for oxygen between myoglobin and mitochondria determines oxygen penetration beneath the meat’s surface, which significantly affects the intensity of surface color. Partial pressures of oxygen greater than that in the atmosphere will facilitate a thicker OMb layer on and just below the meat’s surface. Under anaerobic conditions, DMb will also turn red (Figure 1, reaction 5) when exposed to CO; this reaction is reversible, but the forward reaction is favored.
Deoxygenation of OMb to DMb (Figure 1, reaction 3) is favored under low-oxygen partial pressures that occur when dissolved oxygen in muscle tissue is consumed by various reactions, including mitochondrial respiration. Re-blooming may occur immediately if oxygen re-unites with the DMb. However, DMb is susceptible to oxidation by oxygen radicals and reactive oxygen species (mainly hydrogen peroxide), forming MMb (Figure 1, upper right branch of reaction 3). This reaction occurs most rapidly at oxygen partial pressures of 1 to 25 torr because there is not sufficient oxygen to bind to available DMb. As a result, DMb will react with hydrogen peroxide and oxidize to MMb. Conversely, at oxygen partial pressures that promote DMb oxygenation, there is less DMb available to react with hydrogen peroxide. Thus, greater OMb levels will minimize MMb formation.
Thermodynamically, OMb is resistant to oxidation to MMb; thus, reaction 2 (Figure 1) is unlikely. The rapid browning that often occurs in meat seems to contradict this chemistry, but the origin of MMb is through the deoxygenation reaction of OMb to DMb, which can be rapidly oxidized to MMb. Under aerobic conditions, metal ions (iron, copper) stimulate the formation of oxygen radicals from diatomic oxygen, leading to MMb formation. Metal chelators (such as citrate, phosphates, etc.) inhibit or delay MMb formation. Radical scavenging antioxidants (tertiary butylhydroquinone [TBHQ], butylated hydroxytoluene [BHT], butylated hydroxyanisole [BHA], vitamin E, spice extracts, and plant polyphenols) also slow MMb formation.
Oxidation of ferrous DMb to ferric MMb causes brown discoloration. MMb formation tends to initiate beneath the surface between the superficial OMb and interior DMb where oxygen partial pressure is not high enough to oxygenate all available DMb. Thus, some DMb is available to react with oxygen radicals, forming MMb. Hydrogen peroxide and oxygen radicals are continually present in aerobic conditions because they are by-products of mitochondrial metabolism and lipid oxidation. The thin subsurface layer of MMb thickens as MMb concentration increases. Gradually, the surface OMb layer becomes thinner as the underlying MMb band thickens, encroaches, and replaces the OMb layer to the point that, visually, the surface color changes from bright red to dull red to brown. Conditions that delay the appearance of subsurface MMb include low temperature, high pH, antioxidant capacity, and greater reducing activity. MMb reduction influences meat color stability by regenerating ferrous myoglobin. However, this reaction depends on oxygen scavenging, reducing enzymes, and the nicotinamide adenine dinucleotide (NADH) pool, all of which are limited and continually depleted in postmortem muscle. MMb reduction by endogenous reducing systems in meat may offer a critical strategic approach to decrease MMb formation and increase fresh meat color life.
Mitochondrial activity, enzymatic- and nonenzymatic processes can reduce MMb to DMb (Figure 1, reaction 4); this reaction is critical to meat color stability. Numerous extrinsic and intrinsic factors affect this reaction, but oxygen consumption, MMb reducing activity (MRA), and the postmortem pool of NADH are significant variables in the extension of the color life of meat. Research indicates that the addition of various glycolytic and Krebs cycle intermediates such as glutamate, lactate, malate, pyruvate, and succinate can regenerate reducing equivalents and extend fresh meat color stability.
Visual, practical meat color versus actual pigment chemistry
In the meat industry, meat color chemistry can be confusing because visual observations of color change differ somewhat from the chemical pathways described earlier. Industry practitioners and meat scientists conducting research with meat and meat products usually see brown MMb forming directly from bright red OMb. Thus, it is sometimes difficult to put the principles shown in Figure 1 into practice, especially when troubleshooting meat color problems. In particular, Figure 1 shows that purple DMb is an intermediate in the conversion of OMb to MMb, but this is seldom observed in practice. Rather, Figure 2, reaction 2a shows that bright red OMb changes directly to brown MMb, without any visual development of purple DMb.
Schematic of the visual, practical interconversions of myoglobin redox forms in fresh meat. Myoglobin interconversions in a purified and meat system are different (Figure 1). Figure 2 explains myoglobin interconversion in an in situ meat system and may not be applicable to a purified system. Courtesy of M. C. Hunt, Kansas State University.
Reconciling the apparent contradiction between the chemical and visual pathways
The answer lies in careful observation of the changes occurring at and immediately beneath the meat’s surface. Fresh-cut meat surfaces are purple (DMb) because of the absence of oxygen. After several minutes in air, the meat surface is bright red (OMb; Figure 2, reaction 1). A cross-section of the meat would reveal that the red surface layer is < 1 mm thick, and the deeper muscle tissue is purple. After several hours, the red surface layer is typically 2 to 3 mm thick (thicker in muscles with low oxygen consumption and thinner in muscles with high oxygen consumption). After 1 to 3 d at 2°C to 4°C, a thin layer of brown MMb becomes apparent, just below the OMb layer. As previously explained, the brown layer develops because of reaction of DMb with oxygen radicals forming MMb. Because MMb is usually formed more rapidly (Figure 1, reaction 3) than the reverse reaction (Figure 2, reaction 2b; MMb conversion to DMb), MMb concentration increases with time. By several days of storage or display, the thickness of the surface OMb layer decreases as the MMb layer progressively moves toward the surface, which makes the OMb layer appear duller and dimmer. Eventually, the MRA of the tissue in the OMb layer is depleted and the MMb layer reaches the surface with total discoloration.
How is it known that deoxymyoglobin was formed as an intermediate in the browning reaction?
Metmyoglobin formation is much slower in 70% to 80% oxygen compared with atmospheric conditions (21% oxygen). Thus, the OMb cannot react with oxygen radicals to form MMb. In addition, there is a dynamic disassociation equilibrium in which OMb is continually converted to DMb + oxygen and vice versa. In the brown MMb layer where oxygen levels are low, some DMb has re-associated with oxygen radicals instead of oxygen, causing fairly rapid oxidation of DMb to MMb.
If deoxymyoglobin is formed, why does the surface color change directly from red to brown, with no purple intermediate?
The answer lies in the fact that purple DMb formation is obscured by the overlying red OMb layer during the first 1 to 3 d of storage or display and later by the increasing thickness of the MMb layer. Furthermore, in the surface OMb layer, the small amounts of DMb formed by equilibrium dissociation are rapidly converted back to OMb, owing to the excess of oxygen near the surface.
How does metmyoglobin change to purple deoxymyoglobin after sufficient vacuum (anaerobic) storage?
First, the thin brown MMb layer develops because of vacuum removal of some, but not all, oxygen. The low oxygen level at the meat surface favors browning, as previously explained. The purple DMb becomes apparent only after the overlying red OMb and brown MMb levels disappear. Oxymyoglobin levels go to near 0 mainly because of muscle mitochondrial oxygen consumption. MMb levels go to near 0 owing to somewhat slow enzymatic or nonenzymatic MMb reduction to DMb. It is well known that color-stable muscles do this more easily than color-labile muscles, which may only partially convert MMb to DMb.
The temperature optima for OMb preservation or DMb formation lead to different recommendations for storage temperature. For instance, OMb is most stable at low temperature (−1°C to 2°C). However, DMb will develop more quickly in the OMb–MMb interface area of vacuum-packaged meats if held at warmer temperatures (3°C to 4°C or higher) for several hours, to stimulate mitochondrial oxygen consumption and MMb reduction reactions 2a and 2b of Figure 2.
Meat packaged in aerobic modified atmospheres will also turn brown but at a variable rate depending on muscle, postmortem age (especially at warmer temperatures), and other retail display conditions. Bacterial growth can also affect reactions 2b and 3. Reactions 3 and 4 proceed as described previously.
Why confuse the issue with two fresh color triangles?
In practice, separating the visual conversion of OMb to DMb as shown in Figure 2 with an intermediate formation of MMb allows industry to manage color problems more easily because it separates the required chemistry into 2 critical, practical reactions in which MMb formation (Figure 2, reaction 2a) always seems to occur but MMb reduction (Figure 2, reaction 2b) is often problematic and requires special attention to processing practices.
Factors affecting fresh meat color
The literature clearly documents that many factors affect meat color. Several component traits contribute directly to meat color and the biochemical reactions resulting in changes in meat color. Rate and extent of postmortem pH decline, amount of protein denaturation during conversion of muscle to meat, antioxidant concentrations, biochemical intermediates available to modulate meat color, and the quantity of unsaturated fatty acids directly contribute to multiple mechanisms affecting meat’s use of oxygen and meat’s ability to reduce MMb.
Intrinsic muscle characteristics—such as pH, muscle type, muscle fiber type composition, myoglobin concentration, disruption of various subcellular components related to meat color chemistry, and water-holding capacity—influence the component traits driving differences in lean color and color change during storage or display. Animal genetics interact with environmental factors to determine these muscle characteristics and how they respond to postmortem management. Antemortem factors affecting these muscle characteristics include gender, age, diet energy density, time-on-feed, seasonality, and antemortem stress.
Of the numerous postmortem factors affecting color chemistry, muscle temperature is of the greatest concern. Factors affecting temperature decline during chilling include carcass weight, method of immobilization, chilling rate, and scalding and singeing. Parameters for carcass electrical stimulation have a tremendous impact on the extent of pH decline at a given muscle temperature and therefore are of great importance. Moreover, controlling temperatures during storage, processing, and transport is critical.
Application of antimicrobial interventions, postmortem processing and packaging methods, time and temperature of storage, extent of exposure to oxygen and the number of times meat goes through the color cycle, and postmortem age of the product usually influence meat color by directly and indirectly influencing the component traits of meat color. As many of these factors as possible should be controlled during designing and reporting of meat color research.
Muscle metabolism and meat color
Cellular biochemistry differs across muscles within a carcass, influencing postmortem metabolism of skeletal muscles. McKenna et al. (2005) examined various biochemical mechanisms influencing color stability in 19 beef muscles, whereas other researchers (Mancini et al., 2009; Suman et al., 2009) reported that color-stable and color-labile beef muscles respond differently to modified atmosphere packaging (MAP) systems and to browning induced by cooking.
The role of mitochondria in meat color has received significant attention, and the mechanisms through which mitochondria influence myoglobin redox stability have been investigated. The influences of vitamin E (Tang et al., 2005a), lipid oxidation (Tang et al., 2005a), oxygen consumption rate (OCR; Tang et al., 2005b; Mohan et al., 2010c), and metabolites (Tang et al., 2005c; Ramanathan et al., 2009; Mohan et al., 2010b) on the interactions between mitochondria and myoglobin suggest that both the electron transport chain and reductase enzymes in the outer membrane can reduce MMb and therefore are involved in color stability.
Cooked meat color
The process of cooking denatures myoglobin, which is responsible for the characteristic dull brown color of cooked meat products. However, the denaturation temperature for different redox forms of myoglobin is not constant; therefore, the relative brown color of cooked product interiors is not necessarily a reliable indicator of degree of doneness. Myoglobin’s denaturation temperature depends on the protein’s redox status. More specifically, the relative resistance of myoglobin to heat-induced denaturation is as follows: COMb > DMb > OMb > MMb (Ballard, 2004).
Premature browning (see “Ground Beef Patty Cooked Color Guide” in Appendix B(A)) is a phenomenon in cooked beef in which myoglobin denaturation—and, as a result, cooked appearance—occurs at a temperature too low to inactivate pathogens. Killinger et al. (2000) reported that the incidence of premature browning in ground beef purchased from local retail stores was about 47%. Both intrinsic (myoglobin redox state, muscle source, and antioxidants) and extrinsic (packaging, storage, and cooking from a frozen state) factors influence the susceptibility of beef to premature browning.
Persistent pinking (the opposite of premature browning) is a cooked-color defect in which myoglobin is more stable and resistant to thermal denaturation, resulting in an undercooked appearance. The most common cause of persistent pinking is elevated pH. Slight pinking starts at pH 5.9 and becomes progressively more pink above pH 6. Prevention of pH-induced pinking can be minimized by reducing the pH (logical, but not easily done), increasing the endpoint temperature, or both.
Meat with a normal pH that contains COMb will be more heat stable, increasing the endpoint doneness by one-half to two-thirds of a degree of doneness. Persistent pinking also can be caused by several sources of nitrogen oxides, including small amounts of NO3− or NO2− in other food sources, spices, or water added to meat. Incomplete combustion products of gas-fired ovens also may cause surface pinking. These forms of pinking can be minimized or eliminated by removal of the causative agent and/or increasing the endpoint temperature.
Cured meat color
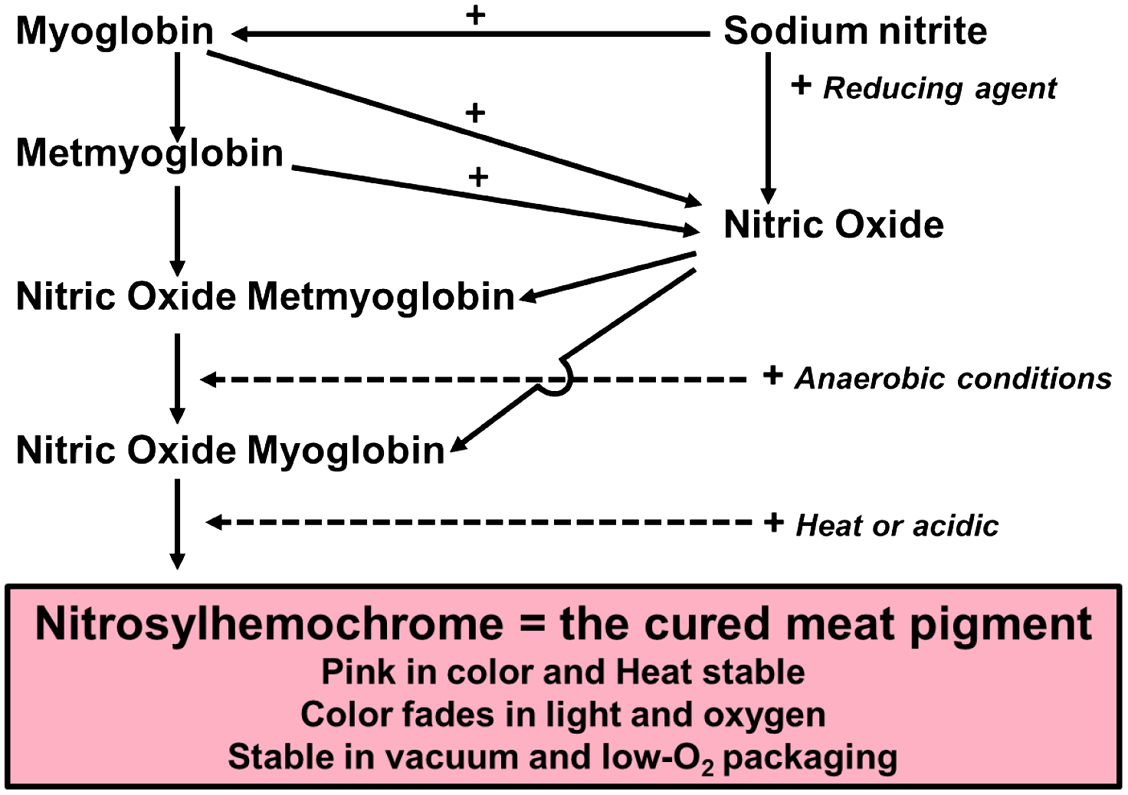
A significant portion of cured meat color chemistry involves the same factors that affect myoglobin chemistry of fresh meat. However, the reactions resulting in the pink, heat-stable, cured meat pigment (nitrosyl-hemochrome) are more complex owing to hundreds of curing protocols practiced globally. Nitrate and/or NO2− are added to many meat-curing formulations in solid or liquid form.
Nitrite addition (Figure 3) causes the characteristic pink color associated with cured products. Added NO2− binds with the heme moiety of DMb, with rapid reduction of the bound NO2− to NO, and simultaneous heme oxidation to the ferric form (Figure 3). Visual indication for this reaction is given by the rapid browning that occurs when NO2−-containing brines are added to fresh meats. Under anaerobic conditions, brown NO-MMb is then reduced to red NO-myoglobin by added reductants such as erythorbate, or more slowly by endogenous reductants, in combination with MMb reductase enzymes. Some studies indicate that in brines containing NO2− and reductants, the NO2− also rapidly reacts with reductants to generate NO, which in turn binds MMb, forming NO-MMb.
Reactions leading to formation of nitrosyl-hemochrome. Note: The solid arrows indicate reactions, and the dotted arrows indicate conditions that favor the reaction. The “+” indicates a reaction between the 2 connected “reactants,” and the product is shown by the next arrow. Examples: myoglobin + NaNO2 can form MMb (this is a step in some MRA assays); metmyoglobin + NO can form NO-MMb; myoglobin + NO can form NO-myoglobin, or with anaerobic conditions NO-MMb is reduced to NO-myoglobin; heat or acid conditions favor globin protein denaturation, and NO-myoglobin is converted to nitrosyl-hemochrome. Courtesy of Drs. M. C. Hunt, Kansas State University, and D. P. Cornforth, Utah State University. MMb, metmyoglobin; MRA, MMb reducing activity.
One precaution in the handling of brines containing NO2− and erythorbate is to keep temperature below 10°C. At higher temperatures, erythorbate will rapidly reduce NO2− to NO gas, which escapes before brine injection, resulting in poor or no cured color development in the cooked product.
Denaturation of NO-myoglobin and NO-hemoglobin during cooking or fermentation exposes the centrally located porphyrin ring, resulting in the pink cured meat pigment nitrosyl-hemochrome, due to the interaction between ferrous iron and NO. The pink color will fade to gray when exposed to light and oxygen.
To cure meat without directly adding NO3−, a variety of non-meat ingredients that naturally contain sufficient NO3− can be added to meat formulations to form a cured color. During processing, one or more non-meat ingredients such as celery juice, powder or concentrates and/or isolates (or numerous other vegetable products, ascorbic acid, cherry powder, spices, etc.) can be reduced so that the NO2− will eventually form nitric acid myoglobin, which will be converted to nitrosyl-hemochrome with heat or acidic conditions. This “cured meat pigment” is pinkish/red, heat stable, and color stable with vacuum conditions. The color fades in air and light (Figure 3). Cured color and flavor stability usually increases with greater amounts of generated NO2−. Meat products cured by this method often are perceived by consumers as being more natural and wholesome. Some regulatory agencies allow these products to be labelled “NO2−-free.”
FreshCase™ (Curwood, Inc.) produces a packaging film embedded with NO2− for use with vacuum-packaged fresh meat (Figure 4). Once packaged, the NO2− is released and forms the uncooked, red pigment (NO-myoglobin, Figure 3) on the surface of the meat. This results in vacuum packages of fresh meat with a reddish surface color rather than the typical purple color of vacuum-packaged fresh meat. Fresh meat packaged with the NO2−-embedded film has greater color stability and shelf life than other fresh meat formats with OMb on the surface. Regardless of the curing methodology used, it is essential that myoglobin be exposed to NO to create several different nitrosyl heme pigments. Thus, optimizing formulations for various extrinsic and intrinsic factors important to fresh meat color chemistry are also essential to achieve desired final product specifications, consistency, quality, and safety of a large variety of processed meats.
What is the actual curing (nitrosating) agent, nitrite or nitric oxide?
There is some disagreement on this point among various literature sources. Recent evidence points to NO2− as the compound that first reacts with heme iron. This makes sense because NO2− is water soluble, with small enough molecular diameter to penetrate the heme cleft, and its negative charge would provide electrostatic attraction to the positively charged heme iron. Nitric oxide gas, on the other hand, is not very water soluble, and tends to leave the brine. Some studies with pure myoglobin solutions, or with meat batters, have shown cured color development after exposure to NO gas. But, NO may not have directly reacted with heme iron in these experiments. Under aerobic conditions, NO reacts with oxygen to form nitrogen dioxide (NO2) gas, which in turn reacts rapidly with water to form the NO2− ion. Thus, even in the presence of NO gas, NO2− is likely the active meat-curing agent. Historically, NO3− salts were used for meat curing but were reduced to NO2− by bacterial action for curing to occur. Cured color development may also occur on grilled or smoked meat, owing to the presence of nitrogen dioxide, which forms NO2− ions when moist meat surfaces interact with combustion gases.
Is cured meat pigment mono-nitrosyl-hemochrome or di-nitrosyl-hemochrome?
Stoichiometric studies found that a ratio of 2 mol of NO2− was needed for formation of 1 mol of cured meat pigment, indicating that the pigment was di-nitrosyl-hemochrome. However, the only study of cured pigment structure using mass spectroscopy found that the molecular ion fragment had an atomic mass of 646 units, rather than 676 atomic mass units predicted for di-nitrosyl-hemochrome. This result strongly indicated that cured meat pigment is mono-nitrosyl-hemochrome. Further work indicated that another NO was bound to the globin portion of the pigment. Thus, 2 mol of NO binds to myoglobin, but only 1 mol of NO binds to the color-inducing heme group.
Iridescence
Iridescence results in a shiny, rainbow-like appearance on the surface of cooked meat products. This kaleidoscope-like appearance is often associated with green, red, orange, and yellow colors caused by product surface microstructure and light diffraction, not the myoglobin redox state. More specifically, structural uniformity on the surface of meat products results in light diffraction conducive to iridescence, whereas disruption of surface microstructure reflects light in a relatively irregular pattern that limits iridescence (Lawrence et al., 2002a, 2002b).
Physics of Color and Light
Perceiving an object and identifying the color of that object involves a complex set of circumstances consisting of the object, its surroundings, and the detector that perceives the object and translates the stimuli into a perception of color. That detector can be the human eye or instrumentation such as a colorimeter or spectrophotometer.
For human sensory response and detection of color, the eye and brain work synergistically to detect and process stimuli to discern color. The eye is composed of the cornea, pupil, iris, and lens, which together form the anterior chamber of the eye. The lens separates the anterior chamber from the posterior chamber (vitreous), which contains the retina and optic nerve. The eye operates much like a camera. Light passes through the pupil where the lens focuses the light onto the retina. The iris works much like a shutter in a camera, opening to allow more light to come into the eye in low light conditions and constricting to restrict light in bright conditions.
The retina is the organelle that senses light. The light detectors of the retina are the rods and cones. Rods are not color sensitive but respond to the visual sensation of light from black through gray to white. The cones are color sensitive to visual sensations of the visible light. The cones can be divided into 3 types based on the portion of the light spectrum to which they have peak responses, blue, green, or red. Therefore, when light penetrates the eye, the rods detect lightness/darkness stimuli, and the cones detect light spectra in the blue, green, and red spectra. The detection of blue, green, and red spectra is referred to as the trichromatic function of the eye. The detection of these stimuli is then transmitted from the optic nerve to the brain, where the information is processed, and a visual perception of the object is developed.
Therefore, the complex interaction of eye and brain is what develops color perception. This is subject to numerous factors that can skew the perception of color, particularly the detection and processing of color. To determine color, a detector capable of capturing this information is necessary. However, not all eyes have the same ability to detect light sensations and process them into color perceptions. As a notable example, some humans suffer from red-green color blindness. Although the light spectrum permits the sensation of color, the detector (eye) cannot detect, and the brain cannot process these stimuli appropriately. Therefore, any color measurement requires a functioning detector. In the case of human color perception, charts for detection of color blindness are available on the internet.
Note that the eye, or some other mechanical device, does not “see” color, it simply captures wavelengths of light reflected from an object, such as meat, and in the case of the eye, relays this sensory input to the brain for interpretation. The color of meat or other objects is the interaction between light, vision (the detector), and the object being viewed. Without light, there is no color and no vision. Visible light is a part of the electromagnetic spectrum, which is defined by the wavelengths of energy and includes broadcast, radar, infrared, ultraviolet (UV), x-rays, gamma rays, and cosmic rays. However, humans can only detect light in the visual spectrum, which ranges from 390 to 750 nm. In this narrow range of the electromagnetic spectrum, the eye has the ability and the brain the capacity to separate wavelengths into color groups. For instance, red color is associated with light of approximately 650- to 700-nm wavelengths. Green color is associated with approximately 490 to 575 nm, and blue is associated with wavelengths between 455 and 490 nm (Figure 5).
For color to be visually detected, light must reflect off the object being viewed and return to the eye. To have color development, the light illuminating the object must contain the spectral range to allow reflectance of corresponding wavelengths that the eye can detect and the brain interpret as color. Therefore, with full visual spectral light comes the possibility for an infinite number of colors to be developed. When light strikes meat, it will be absorbed, reflected, or scattered. The wavelengths of light that are absorbed are not perceptible to the eye because they are retained by the object (e.g., meat; Figure 6). The reflected light is perceived by the eye, captured, and transmitted to the brain. Because the eye is trichromatic, the brain interprets the intensity of the blue, green, and red stimuli that the eye captures and interprets it as a color. So to discern meat color, the source of light must contain the wavelengths capable of reflecting off meat surfaces or color will not be perceptible to the eye or instrumental detector. For sensory and instrumental evaluation of meat, the light source must be standardized. Overall, for humans to see the true color of an object, a balanced light source should be used. In summary, using a light source, such as the incandescent lamp (Figures 6 and 7), will make fresh meat appear more bright red than a 5,000-K fluorescent lamp with a lower red spectral output (Figure 6).
Spectra reflectance of a slice of beef meat (top). Relative reflectance of the different wavelengths (bottom). Please note that the observer sees only the wavelengths/colors reflected from the surface and not the wavelengths absorbed by the surface/meat. Courtesy of Dr. Shai Barbut, University of Guelph.
Examples of spectral power distribution from an incandescent light bulb (left) and fluorescent bulb (right) (https://en.wikipedia.org/wiki/Spectral_power_distribution#/media/File:Spectral_Power_Distributions.png; http://creativecommons.org/licenses/by-sa/3.0/deed.en).
In addition to the physics involved in light detection and color generation and perception, numerous physical conditions impact the color of meat. The following discussion does not focus on the pigment condition or chemistry of meat but how color can be perceived differently for the same cut under different conditions. Conditions that can influence color perception are the light source (illuminant), observer differences, size differences in cut or object, smoothness of the surface (e.g., using sharp or dull knife to cut meat), background differences, and directional differences.
Wavelengths of light reflected from meat develop the perception of color, so the light source becomes an issue in the development and perception of color. Light sources or illuminants come in many different types: sunlight, fluorescent light, and tungsten light, among many others. Even within types of illuminants, lighting sources can differ greatly. Each light source contains a different spectral light composition. Figure 6 illustrates the output of 2 light sources. A so-called balanced light source will have an equal/balanced output of different wavelengths (e.g., sunlight). For this reason, meat may look one way in a retail display case but lose its favorable appearance under store lighting (many stores use fluorescent light in their display coolers because the light bulb produces very little heat and is more efficient than an incandescent light bulb, which loses >70% of its energy as heat). Therefore, when choosing a light source for research, the type of lighting and the light source must remain constant for proper comparison of color. One common light source for viewing meat is deluxe warm white fluorescent lighting. Along with light source, the intensity of light is also important in perceiving color; neither too bright nor too dim is good when viewing meat. Approximately 1,630 lux is commonly used to compare meat samples for color. Figure 8 shows the actual wavelengths reflected from 3 fresh meat cuts illuminated with a cool white fluorescent bulb. These spectra are what would be detected and evaluated by consumers’ eyes. This light bulb has major peaks in the indigo, green, and orange areas (similar to the 5,000-K lamp in Figure 6) but very low output in the red area; thus, a consumer panel perceived the beef, pork, and chicken cuts as brown. In contrast, when the panel was presented the same meat under incandescent lighting (reflectance spectra similar to those shown in Figure 6 and the 2,800-K lamp in Figure 7), the consumer panel scores were pinker/redder.
Relative luminance of fresh meat cuts positioned under cool white fluorescent light bulb. Note that this specific light bulb has major peaks in the indigo, green, and orange colors. The minimal light output toward the end of the visible spectrum results in poor appreciation of the red color of the beef and pork meat cuts. Source: Barbut, S. Effect of illumination source on the appearance of fresh meat cuts. Meat Sci. 2001;59:187–191 (reprinted with permission from Elsevier).
Observer differences are another condition that can affect color perception. Each individual’s eyes are slightly different in sensitivity to color vision. This is perhaps the most difficult to control of all the conditions that affect color perception. Screening for color vision perception can aid in selecting panelists capable of discerning meat color differences (see color blindness charts of Wiegand and Waloszek [2003]; note that a computer screen presentation of these charts might not be correct if the screen is not fully adjusted).
Size differences in meat cuts can also affect how color is perceived because of the amount of light reflected to the eye. For larger cuts, more light is reflected to the eye, and color is often perceived as being brighter and more vivid.
Background differences will also affect color perception. Cuts viewed against a bright background often appear to have duller color, whereas cuts viewed against a dark background often appear brighter. Care should be taken to standardize the background so that comparative color determinations can be made. Also, background color becomes important in meat photography, wherein light backgrounds can give a false impression of dull or pale color whereas dark backgrounds tend to best capture meat color vividness.
In addition, the angle from which the cut is viewed and the incident angle of light from the illuminant source will both affect color perception. This is particularly important when gloss occurs, which may impede ability to view the sample. Furthermore, for conditions like iridescence, the incident angle of light to the observer can render this condition either visible or invisible. Backlighting should be avoided; overhead light is preferred. When setting lights, light intensity should be standardized with a light meter.
Color perception of meat
Once light strikes the surface of meat and is reflected back to the detector (eye or instrument), the processor (brain or microprocessor) interprets color. Communicating color can be quite challenging. To facilitate color communication, tools have been developed to aid in speaking the language of color. The Munsell system, developed by American artist A. H. Munsell, uses color chips to match the color of a specimen. The CIE developed the XYZ tristimulus values (Figure 9) in 1931 and the CIE L*a*b* color space in 1976 (CIE, 1976; Figure 10). The reason the CIE L*a*b* system was developed was that the XYZ colorimetric distances between the individual colors do not correspond to perceived color differences. For example, the difference between green and greenish-yellow is relatively large, whereas the distance distinguishing blue from red is quite small. The CIE solved this problem in 1976 with the development of the three-dimensional “L, a, b” color space (or CIELAB color space). In this system, the color differences one perceives correspond to distances when measured colorimetrically. The a axis extends from green (−a) to red (+a) and the b axis from blue (−b) to yellow (+b). The brightness (L) increases from the bottom to the top of the three-dimensional model (Figure 9). In reporting colorimeter values for research, authors must note whether CIE L*a*b* values or CIE Lab values were used. (The presence or absence of the asterisks is reflective of slight mathematical differences in how each of these values is calculated.)
CIE (1931) color space (illustration of the CIE 1931 color space; https://en.wikipedia.org/wiki/International_Commission_on_Illumination#/media/File:CIExy1931.png; http://creativecommons.org/licenses/by-sa/3.0/deed.en). CIE, Commission Internationale de l’Eclairage.
Perceptible color has hue, lightness, and saturation properties. Hue is the color description as we communicate it in language (red, yellow, green, blue, etc.). Hue is determined by the specific wavelengths reflected from a meat surface back to the detector. Lightness describes the brightness or darkness of the color. Saturation refers to how vivid or dull the color is. To measure or describe color, a number of methods have been established.
The XYZ tristimulus values and the associated Yxy color space established a methodology for describing color (Figure 10). From this, the CIE x, y chromaticity diagram was developed. This representation allowed achromatic colors (pale or dull colors, lower saturation) to populate the center of the diagram, whereas the chromaticity increases toward the periphery of the diagram (more vivid colors, more saturation). Around the periphery are red, green, and blue primary colors and the corresponding wavelengths of visible light associated with those colors. The chromaticity diagram allowed coordinate plotting of x and y color values to determine color (hue) and saturation (vividness).
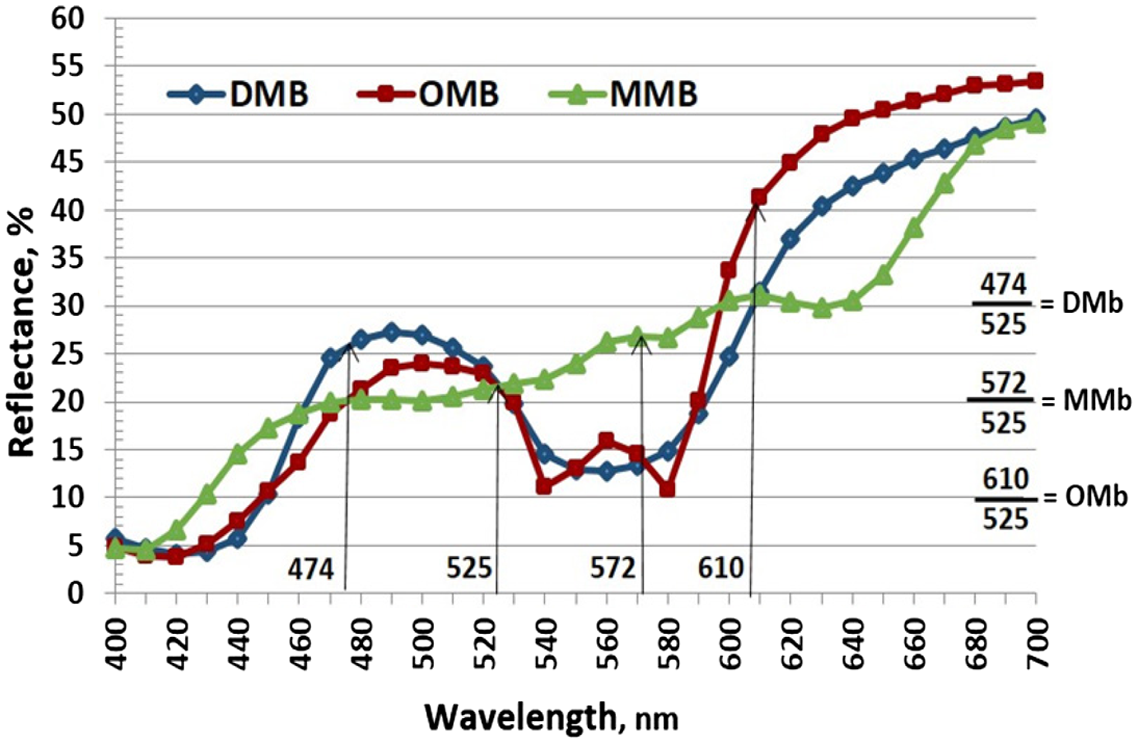
The later development of the CIE L*a*b* color space allowed color to be expressed in a three-dimensional space (Figure 10). Because of the optic response of the human eye to blue, green, and red, calculations converted these responses to L*, a*, and b* values. When combined, these establish a three-dimensional color space. For the color space, a* values are represented on the x-axis, b* values on the y-axis, and L* values on the z-axis (Figure 10). In the center of the color space is neutral gray. Along the a* axis, a positive a* represents red, and a negative a* represents green (scale from +60 for red to −60 for green). Along the y-axis, a positive b* represents yellow, and a negative b* represents blue (scale from +60 for yellow to −60 for blue). The third dimension, L*, is represented numerically in which 100 is white and 0 is black (Figure 10). For this color space, a* and b* values can be plotted to establish the color or hue of a meat sample (Figure 11). Using the L* value, lightness or darkness of the sample can be determined. Therefore, using trigonometric functions, the incident angle that a sample deviates from the x-axis can be calculated to determine the hue angle of the sample, and the distance of the sample from the origin of the XYZ lines can be calculated to determine the saturation or vividness of the sample. Hue angle is calculated as h = arctangent (b*/a*). For example, with a CIE b* value of 14.12 and a CIE a* value of 47.63, the hue angle would be 16.51. Therefore, the plot of the a* and b* points into the hue angle formula will range from 0° to 360° to establish the color of the sample. Likewise, because colors become more vivid around the periphery of the color space, the farther the a* and b* plot points are from the origin, the more vivid the color. Chroma (saturation index) can be calculated from the a* and b* values as (a2 + b2)0.5. For example, with an a* value of 47.63 and a b* value of 14.12, the chroma (saturation index) would be 49.68. With these data, color differences can be calculated and compared objectively.
The physics of light and instrumental color measurements
Instrument packages come in 2 major classes capable of measuring color, the colorimeter and the spectrophotometer. Both use their own light sources and certain illumination conditions (e.g., Illuminants A, C, or D). Various light sources can be used (e.g., tungsten and deuterium). The part of the spectrum and the cost of the light bulb, among other things, influence the decision to use one light source over another.
Instruments differ in the way that they measure reflected light. The tristimulus method uses a light source that illuminates the sample and is then reflected through red, green, and blue filters onto photodetectors (Figure 12). The microprocessor can convert the reflected values to XYZ or CIE L*a*b* values. The spectrophotometer illuminates the sample, and the reflected waves are either scanned (via a monochromator) or read simultaneously by a photo diode array (Figure 13). These values are sent to a microprocessor and can be presented as the reflected spectra and converted to XYZ values as shown in Figure 12 or CIE L*a*b* values.
Some reflectance spectrophotometers are designed to scan wavelengths (colors) reflected from the surface using a diffraction grating, whereas others detect ranges of reflected light via photo diode arrays (a type of photo-detector capable of converting light into either current or voltage, depending upon the mode of operation). A diffraction grating is basically a solid plate with many parallel, closely spaced slits or a plate with many parallel reflecting grooves. Interestingly, a meat surface can also act as a diffraction grating itself. Iridescence seen on intact meat is related to the highly organized structure of the myofibrils within the fibers, so when the surface is cut, it can create a structure resembling reflecting grooves. In that case, the incident light is diffracted (as it would be using a prism) into a variety of hues. However, a spectrophotometer grating can separate the different colors of light much more than a prism with its dispersion effect. Even a single wavelength of light can be diffracted further. Photo diode arrays are designed to simultaneously measure a range of wavelengths. Some photo diode arrays may have a resolution of only 2 to 10 nm; therefore, with a very sharp reflectance peak or valley of interest, a scanning reflectance spectrophotometer may be a better choice. As photo diode arrays are improved, this advantage may be lost. Such high resolution is more pertinent to pigment analysis than tristimulus measurements. Also keep in mind that the scanning reflectance spectrophotometers collect the reflectance over the intended visible wavelength range much slower than diode arrays.
In addition, remember that meat contains multiple hues. For instance, fresh red meat appears red. Although the red hue dominates the spectral reflectance, other hues are also present. A spectral reflection profile is useful to determine the presence of other hues and their intensity. Furthermore, for pigment form, spectral reflectance can estimate pigment form quantities. Both colorimeters and spectrophotometers are useful to track color changes in meat over time because they are non-destructive tests. Important also is that instruments used to measure color vary widely in design features which impact the accuracy and precision of color measurements.
Display Guidelines for Meat Color Research
Purpose of display studies
Assessing meat appearance is a critical step in projecting the retail acceptance of meat products. Beyond meat’s intrinsic properties, many extrinsic factors can affect meat color, and research involving either type of variable often merits a simulated retail display study. The researcher must control all non-experimental factors to accurately discern actual differences due to treatment. The 6 parameters that should be considered, evaluated, and reported for any meat-display study are packaging, handling and storage, lighting parameters, display temperature, display duration, and display case configuration. Color evaluation during display studies typically involves at least one of 2 measurement methods: visual (panelists) and instrumental (colorimeter or spectrophotometer). Display studies may only include a single type of color assessment. If display focuses predominately on instrumental color measurements, it is very highly recommended that some “unofficial” visual descriptions of product color be taken at the start and termination of display so that authors can describe the general state of discoloration.
Packaging materials affect meat appearance
Package functionality
The functionality of any packaging system depends on the inherent, fundamental chemical and physical properties of the meat and their interactions with the various aspects of the packaging system. Hence, the materials and methods should contain antemortem details of the animal’s background, such as genetics, breed, sex, age, and production and nutritional history. In addition, pertinent details, such as the basis for treatment assignment to meat samples and replications, are needed as well as a complete history of postmortem events, such as stunning method, slaughter, electrical stimulation, chilling, carcass interventions, postmortem age, storage conditions of cuts, anatomical location of muscles and cuts removed, and geometric orientation of the muscle fibers.
Packaging materials
The type of film, bags, and trays/lids (e.g., rigid plastic or Styrofoam™) should be described using the manufacturer’s name, product number, chemical composition, thickness, bag size, oxygen and water vapor transmission rates, and—if appropriate—the color and position of the label. Packaging materials used in the study should also be used for training of visual panelists, for standardization of instrumental color measuring instruments, and for the development of myoglobin redox standards that can be used for quantification of myoglobin redoxforms.
Other considerations
The use of soaker pads, oxygen scavengers, or other atypical components should be detailed. Specifications (brand, model, composition, etc.) of additional components should be reported.
Atmosphere
The package atmospheric environment (gases or vacuum) must be considered. Often meat is overwrapped with film that is highly permeable to oxygen, but other formats are available, each with unique film/tray requirements. These should be carefully selected and clearly reported. In modified atmosphere packages, gas concentrations (molar or percentage concentration) should be monitored throughout display because minor variations can significantly affect meat color and color stability. To measure headspace concentrations, a headspace gas analyzer should be used, and the post-packaging time should be recorded.
Vacuum levels
If evaluating vacuum-packaged product, researchers should verify the vacuum level in the packages with a Kennedy Gauge (see Appendix E for information regarding this gauge) or an equivalent device to determine actual in-package vacuum levels. Vacuum levels from gauges near the vacuum pump often overestimate the in-package vacuum level. Researchers should be aware of the oxygen concentrations remaining in head spaces or in vacuum packages depending on the level of vacuum achieved. Even with high levels of vacuum pulled, significant oxygen may remain in the environment and impact MMb formation (see “Partial Pressure” in Appendix E).
Package failure
On occasion, a package may fail (package atmosphere becomes compromised or exposed) during a study. Failure may be the result of product mishandling, lost vacuum, poor seal integrity, or leakage during gas sampling. Be careful not to puncture packages with needles during gas measurements and check the stick-on septa for seal integrity. When visible package failure occurs or when there is an inability to maintain the desired atmosphere composition, that sample should be terminated from the study immediately and all data associated with it removed from data analysis. Because it is impossible to know when such failures occur or what effect the failure may have had on previous observations, an uncontrolled variable is introduced to the study, often invalidating conclusions.
Sample preparation
When preparing meat cuts or products for packaging, take care to standardize the time and conditions of exposure to atmospheric conditions before packaging. After the packaging process is complete, samples should be stored for sufficient time to allow equilibration with their atmosphere to occur (unless changes in the equilibration process are the subject of evaluation). For optimal and rapid blooming in oxygen-containing atmospheres, meat should be held at cold refrigeration temperatures, 0°C to 2°C, for at least 30 min. For adequate deoxygenation or pigment reduction in non- or low-oxygen atmospheres, samples may need to be held for longer periods at slightly warmer (4°C to 7°C; avoid abusive temperatures) temperatures to facilitate enzymatic scavenging of oxygen by the muscle and subsequent MMb reduction.
Microbial considerations
Care must be taken during sample preparation to reduce microbial contamination of product. Researchers should always start sample preparation with clean cutting surfaces and preparation tools. If knives are used, they must be regularly cleaned throughout preparation and rinsed thoroughly before reusing. The researcher and assistants should use clean gloves and change them frequently, taking care to avoid touching the product more than necessary and to avoid touching surfaces that will be evaluated for color and color stability.
Labeling
Each package should be labeled with a random 3- or 4-digit numeric identifier to facilitate panelists’ evaluations and to keep treatments unidentifiable, thus eliminating panelist and researcher bias.
Product handling and storage should mimic real-world parameters
Sample handling and processing should mimic industry practice as much as possible. Aging times should reflect those typical of the product being studied. Current commercial meat production and processing systems are changing to centrally packaged and distributed case-ready products. These products are seldom packaged and placed immediately into display. The complete handling system, especially postmortem age of the product and the time and temperature of storage (dark or lighted) should be described and reported.
Lighting types and intensity affect meat appearance
Light type and intensity will affect how product appears and its discoloration pattern. For most display studies, samples will be continuously subjected to a light source for 24 hours per day for the study’s duration. In addition, these studies should occur in a room where non-display lighting is not a factor and outside light sources are eliminated. However, if the display mimics retail store conditions, room lighting similar to lighting in retail stores would be appropriate. In this instance, case lighting and room lighting should be clearly detailed and reported. In the case of dual lighting situations, visual evaluations should be standardized for all samples and all panelists. Fluorescent, halogen, high-intensity discharge, incandescent, and light-emitting diode (LED) lights are all possible light sources for store and display lighting.
Even though meat may be viewed by consumers and researchers in a variety of different environments, meat research display studies should use either LED or fluorescent lighting. In most countries, fluorescent lighting is being converted to LED lighting because of less heat generation and more operating efficiencies. Because there is a wide availability of fluorescent and LED lighting, meat researchers must know that all fluorescent bulbs and LED lighting are not identical or interchangeable. Each type has its own individual properties, which affect meat color and color stability (Figures 14–15). Correctly selecting light sources for meat research, and reporting light source characteristic data, is critical.
This image depicts the various colors of light produced by different fluorescent bulbs lighting a coffin meat-display case. Note the 5 different colors, all indicating the need to remember that all fluorescent bulbs do not have the same color temperature and other properties. Courtesy of C. R. Raines, The Pennsylvania State University, and M. C. Hunt, Kansas State University.
This picture depicts the exact same unpackaged cuts of beef, pork, chicken, and hard salami under various fluorescent lighting types with different color temperatures and color rendering indices (see Meat Pictorial Guides E, Meat Lighting Facts of Appendix B). Courtesy of C. R. Raines, The Pennsylvania State University, and M. C. Hunt, Kansas State University.
Ideal meat-display lighting
Investigators should use only one bulb type per research study, unless lighting type is being studied. Lights in display studies should have a color temperature of 2,800 to 3,500 K and a color rendering index of 80 to 90. Lighting intensity should be between 1,612.5 and 2,152 lux (150 to 200 foot-candles; 1 foot-candle = 10.76 lux) as measured by a light meter at the meat level. Light intensity should be measured at multiple locations within the display case, including the sides. Front and back areas catch reflections, which can influence color stability. Lights should be adjusted above the displayed meat to produce a meat-level light intensity within this range. Dimmers and timers can be useful for simulating light intensities and exposure times at retail display.
Lighting with attributes to avoid
Cool white fluorescent bulbs are too blue and green (Section E, “Meat Lighting Facts,” in Appendix B). Similarly, color temperatures of 4,000 to 6,500 K are too blue and will not adequately represent meat color. High-intensity discharge bulbs may make meat products appear yellow. Lamps with greater output of UV light and short-wavelength visible light (blue, violet, and green) can accelerate discoloration of some fresh and/or cured meat products. However, UV light was about 2% of the total light and was less damaging than the short-wavelength visible light (Böhner and Rieblinger, 2016). Incandescent bulbs have an acceptable color temperature but may provide uneven illumination and excessive heating of the product, thereby accelerating discoloration.
Display temperature affects color life
Display temperature significantly influences meat color stability. Typically, the reported display temperature for meat color studies has been < 4.5°C. However, in many countries, retail case temperatures run higher under normal operation. In addition, case temperature will fluctuate and may include case defrost cycles sometimes exceeding, albeit temporarily, 10°C. If the display is in a cooler without a defrost cycle, this should be stated clearly because that is not a standard procedure in many retail stores. Regardless, continuous temperature monitoring is necessary to ensure the target temperature is achieved and maintained.
When setting up experimental procedures, researchers should select a display temperature appropriate to the goals of the research, which could be either ideal or abusive. Temperature control is essential if detecting true treatment differences in color or color stability is the goal. The recommended average display case temperature is 0°C to 2°C for non-abusive display temperature research. Investigators should distribute temperature loggers in various locations (front to back, side to side) of the case to continuously monitor air temperatures at the meat level. If multiple cases are involved, standardize the cases for temperature and monitor temperature profiles at identical locations within the cases prior to display.
When insufficient product is available to fill the retail case, investigators should consider filling the cases with packages of salt water (just enough salt to keep the water from freezing) to simulate a full case of meat. An appropriate number of defrost cycles for the display temperatures should be selected. Investigators should fully report the frequency, duration, and extent of the defrost cycles. Color evaluations (visual or instrumental) should not be scheduled during defrost cycles because some packages may “sweat” or form excess condensate inside the package during defrost. Packages displayed at a slight angle on the shelving allow condensate to flow to the edge of the package, without dripping on the product, thus preventing artificial discoloration.
Researchers must manage their samples carefully during display studies. Cases and product should be checked at least twice a day, if not more, to ensure temperatures are maintained within the specified range. During the display period, package location must be rotated within the case by randomly repositioning packages throughout the case once or twice daily. This will help reduce variability due to temperature and/or lighting intensity differences within the display case. In single-level (coffin) display cases, packages should be rotated daily to minimize within-case location effects from front to back and side to side within the case(s) during the display. Product rotation from one shelf to another shelf in multi-tiered cases is not recommended because lighting and temperature will vary across shelves. Thus, product rotation within a shelf is recommended using some scheme for side-to-side, front-to-back rotation. A study in which case temperatures are too high or too low results in erroneous data, lost research product, and unrecoverable time. Display studies are never a maintenance-free endeavor. If there are not enough test packages, other dummy packages, such as small freezer bags, should be used to mimic a full case of product.
Meat color evaluated against time to determine meat color stability
A primary objective of display studies is to evaluate color deterioration (or maintenance) over a given time period. Display studies can last a few hours to several days, weeks, or even months. Investigators need to determine an expected “end point” either before the trial begins or as it proceeds based on the color stability of the product(s) being evaluated. When scheduling a study, investigators should include extra days should product “last” longer than anticipated. Conversely, differences in color stability may be apparent short of the originally planned timeframe. The probability of either scenario can be limited by conducting a short, pre-trial study using factors of the main project. Panelists should be instructed to not handle packages or move them during display so differences in viewing angle or distance among panelists are not introduced.
Configuring a meat-display case
Meat-display studies can be conducted in single-level or multi-level display cases. The requirements discussed previously remain the same. Single-level cases make it easier to manage light intensity, keep visual distance from the product consistent during evaluation, and avoid tier-to-tier temperature variation. However, if multi-level cases are used, temperatures and light intensity at the meat level on each tier within the cases should be monitored and reported.
Occasionally, display cases of either configuration type may not be available for color studies. If this occurs, large, refrigerated rooms (e.g., walk in coolers) may be used. However, researchers must construct lighting structures and display surfaces that maintain consistency for light, temperature, and product. Light sources within the cooler other than the display lighting must be eliminated. People coming in and out of the cooler must be minimized to reduce temperature fluctuations and outside light. Under such conditions, researcher vigilance is even more important for monitoring the meat-display conditions.
Display factors to report
Numerous items have been presented, and nearly all of them should be reported in articles containing data for a simulated meat color stability display study.
Visual Appraisal Principles for Meat Color Measurement
Visual appraisals of color are the “fundamental standard” of color measurements because they closely relate to consumer evaluations and set the benchmark for instrumental measurement comparisons. Like all sensory evaluations using human panelists, visual color panels are not easy to conduct because human evaluation may not be replicable from day to day and is influenced by personal preference, lighting, visual deficiencies of the eye, and environmental appearance factors other than color. Moreover, meat color cannot be stored, maintained, or reliably reproduced over time. Yet, through proper panel management, sample presentation, and data collection procedures, visual appraisals of color can provide accurate and repeatable objective data. These guidelines will provide a brief overview of key concepts that must be understood and practiced when preparing to conduct sensory studies, including visual color panels or studies using only instrumental color measurements.
Conducting research using human panelists
Before sensory work is initiated, most educational, research, and governmental entities are obligated to contact their institutional review board (or similar group) to obtain approval of the details and protocol as mandated by federal laws and regulations for the oversight of all activities involving research with human subjects. Obtaining proper informed consent of panelists is part of this approval process. There are very few exceptions to the requirement for obtaining this approval. Some scientific journals also require evidence of the proper use and approval of human subjects in research. Do not wait until the last minute for obtaining these approvals because there may be several levels of approval needed.
Key concepts for conducting color research using human panelists are presented in Table 1. These guidelines provide only a brief overview of sensory techniques as they apply to evaluating meat color. More detail on sensory methods are in the American Meat Science Association Research Guidelines for Cookery (AMSA, 2016), Sensory Evaluation, and Instrumental Tenderness Measurements of Fresh Meat, in ASTM (ASTM Committee E-18, 1968a, 1968b, 1978, 1979, 1981) and IFT publications (IFT Sensory Evaluation Division, 1995), as well as Meilgaard et al. (1991) and Miller (1994). These documents focus primarily on sensory methods for flavor and tenderness evaluation but provide extensive guidance on training and conducting sensory panels, much of which applies to visual panels as well. Thus, these documents should be thoroughly reviewed before initiating visual color evaluation studies. Additionally, these documents highlight what information should be provided when publishing sensory research. A list of such information is presented in Table 2.
Key steps to conducting trained descriptive visual color research panels
| Item | Description |
|---|---|
| Use human panelists | Gain the appropriate approval(s) for use of human subjects in research |
| Select panel type and appropriate scales | The panel type and scale should appropriately address the objectives of the experiment. |
| Identify panelists | Panelists should have normal color vision and acuity, which should be assessed with the Farnsworth-Munsell Hue test. Select a panel leader. |
| Conduct preliminary trial | A small preliminary trial should be conducted on samples treated in accordance with the experimental protocol. |
| Scale refinement | During the preliminary trial, scoring scales can be adjusted to reflect changes observed in samples during the preliminary trial. |
| Panel orientation/training | During the preliminary trial, panelists should be oriented to the scales and trained to score samples equally. |
| Conduct the experiment | Panel viewing conditions should be standardized. |
| Monitor panel performance | Panelists’ scores should be monitored in reference to panel leader scores. Preliminary analyses including panelist × treatment interactions may indicate shortcomings in panel performance. Panelists identified as not performing adequately should be excluded and/or retrained. |
| Statistical analysis | Average panelist scores and apply appropriate statistical models. |
Information that should be reported in scientific reports with trained descriptive visual color panel data
| Item | Description |
|---|---|
| Type | Consumer or trained |
| Panel selection criteria | Normal vision, acuity, prior experience, etc. |
| Number of panelists | Minimum number of panelists each day (if different from total) |
| Training | Number of sessions, standards used, pictorial standards (if used), etc. |
| Display and viewing conditionsa | Lighting, packaging, and other pertinent factors; see Display guidelines |
| Session descriptions | Days of display evaluated, number of samples per session, time of day if varied, etc. |
| Scales | Establish anchors and descriptors in allowed increments (if applicable) |
| Statistical methods | Experimental design and statistical analysis |
This information should be reported if different from the display/storage conditions.
Types of visual panels
Color panels can be broadly classified as trained visual color panels or consumer panels. Trained, descriptive visual color panels are most commonly used in meat color research and can be regarded as objective instruments. Trained descriptive panelists undergo rigorous screening and training to obtain quantitative ratings of samples on anchored scales. These panelists should not be asked to rate personal preferences or acceptability of the samples they evaluate. Consumer panelists, on the other hand, are useful for providing information using hedonic scales of their preferences and the acceptability of the product’s attributes. The particular research question determines which type of panel can provide data that address that research question. To fully address all pertinent questions, using both types of panels may be appropriate.
Selecting panelists
Consumer panelists
Consumer panelists are generally recruited from predefined demographic groups based on the population of interest. For example, a consumer panel made up of 18- to 21-year-old college students may not provide responses representative of older, more affluent professionals being targeted by branded programs. It may be advantageous to target panelists that meet certain criteria, such as being the primary grocery shopper or food preparer in their family. Consumer panelists generally are given only basic information required by informed consent regulations and receive no training other than instructions in completing the ballot or questionnaire. Consumer panels may be conducted by allowing panelists to rate products on their own in a home environment, which provides consumer perceptions in the environment in which a product is to be used. However, this approach is prone to data recording errors and incomplete results. Alternatively, panelists may be brought to a central location and presented products under controlled conditions with researchers available to help record data. Such “capture panels” allow more correct and complete data, but resulting consumer perceptions fall outside “typical use” conditions. Regardless of location, a sufficient number of panelists must be recruited to avoid bias. The number required will depend on the products and criteria to be evaluated, but a rule of thumb is that a consumer study should involve at least 100 consumers.
Trained descriptive visual color panels
ASTM-434 (1968b) suggests a minimum of 5 panelists, because using fewer than 5 depends too much upon any one individual’s response. Typically, a minimum of 8 panelists are used to evaluate each sample, though otherwise unsuitable panelists should not be used simply to meet an arbitrary number of panelists. Because color panels are generally conducted over many days, a larger panel may be beneficial so panelists’ other obligations do not prevent the required number of observations being obtained.
Training panelists
At a minimum, trained descriptive panelists should be recruited and initially screened based on availability, interest, and normal acuity (such as not being color blind), and they should be able to discriminate color differences using a Farnsworth-Munsell 100-Hue test (see Appendix E for more information). The Farnsworth-Munsell 100-Hue test can be taken online at http://www.xrite.com/custom_page.aspx?PageID=77andLang=en. Successful panelists should have a score of 50 or less if possible (prospective panelists with scores of more than 100 should not be used). Kinnear and Sahraie (2002) reported that panelists between ages 14 and 59 y scored better on the 100-Hue test than those outside this age range. Further training should confirm panelists’ ability to provide accurate and repeatable data using an anchored scale. During this time, the lead investigator or other highly experienced person should serve as the panel leader, providing guidance to panelists on the scale and ensuring that panelists score samples equally. A pre-trial orientation for panelists should include discussions of time requirements; projected dates of evaluation; orientation to packaging, display conditions, and data sheets; and a discussion of color descriptors. A preliminary trial also provides an excellent opportunity for panel orientation and training, as well as any necessary adjustment to the scales being used. Panelists generally should not be aware of the treatments being studied unless that information would help them adequately assess samples. In any case, panelists should not be aware of the treatments to which individual samples belong.
Scoring scales
The relevance of the results of color research conducted with trained descriptive visual panelists relies heavily on the suitability of the color scale. The scoring scale must be properly constructed to obtain data that characterize differences (or lack thereof) between experimental treatments. Thus, the color scale itself must address the correct research questions to be useful. An ideal scale for characterizing discoloration of fresh beef steaks will be of little value in characterizing the fading of cured, frozen pork chops. Furthermore, some scales ask the panelists to provide an “average” color value for an entire sample, whereas others specify the “worst-point” color. Both approaches are informative but yield different results, and investigators must decide which approach will give results most relevant to a particular experiment and the question that experiment attempts to answer.
Panelists need to know whether they will be evaluating initial color and/or color change during display. These require different scoring scales. Often the initial color is characterized at the beginning of display, whereas the color change is evaluated using discoloration scales at the beginning of display and various storage/display times. If percentage discoloration (due to any “deteriorative color” or to MMb, specifically) is evaluated, be sure that the percentage breaks in the scale are realistic and reflective of consumer discrimination. Research generally shows that consumers start to detect and discriminate against surface MMb levels 15% to 30% of the total area, but the area covered by the discoloration needs to be carefully categorized by percentages (for examples of discoloration scales, see Appendix A).
When possible, pictorial aids should accompany the scoring scales (see Appendix B for example pictorial aids). Photographs taken during pre-trials before the actual experimental study begins are particularly useful as a reference for subsequent scoring scale refinement and during panelist training and practice sessions. Pictorial color standards should be stored in the dark because most are subject to light-induced color changes. Often, high-quality photographs of meat that are very representative of OMb, DMb, MMb, good cured color, and typical faded cured color are extremely useful. These scales and pictures are provided because they have been used successfully in research trials and can serve as a template for designing scales in future research. However, investigators should note that conditions unique to each experiment (such as, e.g., display temperature, postmortem age, frequency and duration of defrost cycles, lighting intensity) as well as experimental treatments will alter changes observed during any given display study. Therefore, conducting preliminary trials is best, with meat treated as prescribed by the experimental protocol. In this way, the selected scale can be compared with observed changes in color and adjusted as necessary. Furthermore, researchers should note that hedonic scales appropriate for consumer panels differ in aim and scope from the quantitative scales appropriate for trained laboratory panels.
Sample presentation
Regardless of the type of panel, results depend greatly on sample presentation and the conditions under which samples are presented. As is the case with any analytical technique, color evaluation must overcome the fundamental problems of obtaining a representative sample. Sample preparation for color measurement requires standardized procedures that are both repeatable (by the same person in the same laboratory) and reproducible (by different people in different laboratories at different times). All samples must be handled in the same manner to prevent artifacts. This is particularly important when live animal treatments are evaluated for their effects on meat color. Factors for which standardization is especially important include (unless the factor is an experimental variable) animal nutritional regimen, carcass chill rate, muscle, sample location within a muscle, muscle fiber orientation, muscle pH, time and temperature of postmortem storage, muscle exposure time to oxygen, marbling content and distribution, surface wetness and gloss, myoglobin concentration, packaging, and display conditions (see the previous discussion of display conditions for more details).
Color viewing conditions
Presentation conditions are critical to sensory evaluation. The environment should be free of distractions. Panelist fatigue can affect the accuracy and repeatability of evaluations, so the number of samples must be reasonably limited. The number of samples that panelists can score in a single session will be greatly influenced by the number and complexity of attributes to be evaluated. Because perceived color depends on light source and viewing angle (see earlier review of the physics affecting meat color), these factors must be standardized. Meat color evaluation panels are often conducted with products in simulated retail display. Thus, the display environment must be conducive to panel data collection. For studies evaluating color stability during display, all panelists should be asked to score samples within a small time window (e.g., between 0900 and 1100) on each evaluation day. Sample evaluation should be timed to avoid defrost cycles, because condensation may form in the packages and hinder proper evaluation as described earlier.
Sample identification
Sample identification numbers should be a randomly assigned, 3-digit number that does not indicate treatment group or subconsciously introduce other bias. For example, a panelist may subconsciously give higher scores to a sample identified as number 1 than to a sample identified as number 2. Numbering systems should blind panelists to treatment assignments. This is particularly important in the case in which investigators familiar with the treatments must be pressed into service as panelists.
Monitoring panelist performance
Once a laboratory panel has been trained and an experiment has commenced, the performance of the panel as a whole, as well as individual panelists, must be monitored over time. Individual panelists’ scores should be plotted daily in reference to the panel leader’s scores. Panelists whose scores systematically differ from the panel leader should be retrained. Between replicates (or at least prior to the final statistical analysis), conducting a statistical analysis with panelists in the model can be useful in evaluating the performance of the panel. A significant panelist × treatment interaction indicates that one or more panelist is not performing adequately. Excluding these panelists until they receive additional training could be considered.
Electronic scoring for visual meat color
Tablets and other electronic devices are recommended for panelist scoring of meat color, especially display studies over time. There are numerous software packages available for these units.
The major advantages of electronic data collection include the following: eliminates the use of paper; eliminates error-prone hand input of data; speed, accuracy, and legibility of the scores; exact tracking of sample numbers with the sample’s panelist scores; insurance that all data were entered before downloading of panelist data; monitoring of panelists; greater ease of archiving data; and the ability to use the entire electronic system for other data collections. The major disadvantages are the initial cost and the need to adapt third-party software to the researchers needs.
Statistical analysis
Generally, individual panelist scores should be averaged for statistical analysis, because other methods depend too much on individual panelist observations. Traditionally, visual panel data have been evaluated using standard analysis of variance techniques. Such analyses must account for the covariance relationships between observations taken from a single animal/subprimal over time. Depending on the experimental design, this often entails repeated measures or split-plot models. Though less commonly used, nonparametric approaches such as principal component analysis may provide insight into relationships among color attributes and treatment factors difficult to obtain from analysis of variance.
Summary of conducting color panels
Using human panelists to evaluate meat color attributes is a powerful tool in meat color research. However, effective data collection poses significant challenges to researchers that can compromise the quality of the resulting data whether using trained panelists or consumer panels. Understanding the principles of sensory analysis and following these guidelines will allow researchers to maintain the integrity of their color panel data. Complying with these suggestions will make the peer-review process go more smoothly in publishing meat color research using color panels.
Instrumental Meat Color Measurement
Instrumental color measurement is an objective color characterization method that works well alone or in combination with visual color data. These guidelines can be used as a quick reference for collecting instrumental color data. However, for more information regarding the fundamentals of instrumental color measurement, see the previous discussion on the physics of color and light. For more specific information regarding instrumental methods to quantify myoglobin redox forms, see Appendix C.
Instrument selection
Instruments for meat color measurement are classified as either colorimeters or spectrophotometers. Colorimeters only measure tristimulus values (such as CIE L*a*b*) and often have a set combination of illuminant and observer. A new, relatively inexpensive colorimeter utilizing NIX™ technology (Hodgen, 2016; Holman et al., 2018) merits more study for color measurement of meat and meat products. Spectrophotometers are more complex instruments that supply spectral analysis in intervals of 1 to 10 nm and offer several illuminant/observer combinations for the calculation of tristimulus values. Both instruments are excellent for meat color measurements, but for estimating percentage of surface myoglobin forms, a spectrophotometer must be used.
Illuminant selection
Illuminants are the light source utilized by the colorimeter or spectrophotometer. Investigators must decide on the best illuminant based on the type of sample being evaluated. The most commonly used illuminants in meat color research are A, C, and D65.
Illuminant A (average incandescent, tungsten-filament lighting, 2,857 K) places more emphasis on the proportion of red wavelengths and is recommended for samples for which detection of redness differences between treatments is the priority. Values of a* measured for Illuminant A will be larger than those for Illuminant C (average north sky daylight, 6,774 K) and Illuminant D65 (noon daylight, 6,500 K). Illuminant A is recommended for measuring meat color.
Illuminants C and D65 place less emphasis on the red wavelengths and are frequently used to evaluate many (non-red) food products. Small differences in redness may not be as easily detected with these illuminants, yet the relative differences detected should be in the same order as those obtained from Illuminant A. Values for a* from Illuminants C and D should be similar in magnitude but considerably smaller than for Illuminant A. Some laboratories have instruments without Illuminant A capability, and some laboratories have a history of using either of these 2 illuminants; in either case, the continued use of Illuminants C or D65 is logical.
Other illuminants, F (several in a fluorescent series) and D (several in a daylight series), are available and may be appropriate for some meat investigations. If other illuminants are used, it is recommended that some appropriate comparisons be made with Illuminants A, C, or D65.
Values for a* can vary by 5 to 25 units for the same sample depending upon the illuminant used. For beef with a bright red, “bloomed” color, typical a* values for Illuminant A are 30 to 40+, whereas a* values for Illuminants C and D are 20 to 30+. However, this also depends on the aperture size; smaller apertures will further reduce these values.
Investigators should conduct a literature search, use information from instrument suppliers, and consider the sample properties when selecting the illuminant to use for instrumental color evaluations. We strongly suggest that researchers consider using Illuminant A as the illuminant of choice unless the product you are studying already has an illuminant requirement. Some instrument companies provide software to interconvert between illuminants, but it may be necessary to collect reflectance data from 400 to 700 nm for these interconversions.
Degree of observer selection
Some instruments provide multiple degrees of observers (see “Standard Observer” in Appendix E). Most common are 2° and 10° observers. The 10° observer is most commonly used for meat color measurement and is recommended because it captures a larger portion of the sample scanned, and it aligns with the CIE 1964 10° Standard Observer.
Aperture size selection
Selecting and reporting aperture size for examining meat color is often overlooked but is vital in interpreting data and comparing data among studies. Researchers often attempt to compare their color data with those of other researchers without due consideration for differences related to aperture size. This frequently results in erroneous comparisons. As aperture size decreases, the percentage reflectance decreases particularly at red wavelengths between 600 and 700 nm (Yancey and Kropf, 2008). This can also affect reflectance ratios like the 630/580 nm ratio, which describes meat discoloration, and the 650/570 nm ratio, which describes cured meat fading. Additionally, tristimulus CIE L*a*b* values decrease as aperture size decreases, with the most difference noted in a* values.
Selecting an appropriate aperture size is inherently associated with the size and texture of the sample being evaluated. Aperture sizes can range from 8 mm to more than 3.18 cm. Select the largest aperture size that allows multiple measurements (at least 3 should be taken, and 5 to 10 are recommended with smaller apertures) of the same sample. If samples have a non-uniform appearance (e.g., samples with high quantities of intramuscular fat or connective tissue), investigators should select the aperture size that covers only the meat area and use >3 scans per sample, then average the values.
Researchers must not change aperture sizes during an experiment because values for CIE L*, a*, and b* will differ across aperture sizes. Moreover, regardless of the instrument used and the color variable being measured, emphasis should be placed on the quality of each scan ensuring that the measurements are reasonably close together. Means for L*, a*, b*, chroma, and hue angle that are more than ± 2 standard deviations could be used as a rule of thumb to detect outliers.
Instrument standardization
Instrument standardization and re-standardization are critical for reliable data collection. Standardization of instruments may vary by model and brand. Thus, following the direction supplied with the unit is essential. Generally, standardization is based on scans of black and white standardized tiles. Investigators should follow this at start up and when re-checking standardization periodically, especially if the environmental temperature varies where measurements are taken.
Before standardizing the instrument, determine the type of packaging materials that will be used and retain some unused samples for use in standardization. For example, if the meat samples are packaged using polyvinyl chloride film, the standardization tiles should be wrapped in that film. Ensure that this film is pulled smoothly about the tile, that it is not wrinkled, uneven, or smeared with fat or protein, and that the film is changed frequently to eliminate inaccurate standardization. Standardization procedures should be reported in articles.
Sample thickness and uniformity
Usually, samples at least 12 to 15 mm thick are sufficient to absorb non-reflected light. Translucency of samples should be checked by holding the instrument on the sample in a dark room and watching for light to pass through the sample. If light passes through, then a standardized white background must be placed behind the sample (black backgrounds are harder to standardize than white). Wafered product or other thin samples should be stacked to a uniform thickness and then put on a white or black tile or other background such as Styrofoam or other packaging trays.
Areas within a sample can vary in size, color, and structural uniformity, so sample surface uniformity must be considered. Specific areas can be severely discolored and contain intra- muscular fat or seams of connective tissue, whereas other areas have normal color. With larger aperture sizes, 3 to 10 scans are recommended. More scans are appropriate if the sample has sufficient size to allow multiple scans or varies considerably in color across the surface. Multiple scans may then be averaged for statistical analysis if all scans used the same aperture size.
Protecting the aperture port
Meat surfaces with considerable moisture (as in pale, soft, and exudative meat or enhanced treatments) may create problems with light reflectance and accurate readings. Effects of excessive surface moisture can be minimized by uniformly blotting the moisture from the surface. Some instruments have a glass port covering the aperture opening. Care should be taken to removed any condensation or haze on the inside of the glass cover and keep fat smears off the outer surface. If the aperture is uncovered, moisture can be prevented from entering the instrument’s reflectance port by taping a piece of thin film such as polyvinyl chloride or a piece of spectrally pure glass over the instrument’s port. Standardization procedures should include such a covering if it is to be employed in the scanning of samples. Researchers should take care to remove moisture and fat smears from this surface after every scan. Films should be changed frequently, especially if bottoms of samples are scanned (reflectance unit beneath the sample). Cleaning the interior of the port is best done by a specialist.
Two-toned versus discoloration pattern
Most large muscles of the bovine hind leg and some quadriceps muscles have two-toned muscle color due to differential chilling rates, pH declines, and/or muscle fiber type. These muscles routinely exhibit a two-toned appearance in the superficial versus the deep portions and should be analyzed as “separate” muscles (see Sammel et al., 2002a). Instrumental color scans should be taken of each muscle area and averaged independently.
When samples do not exhibit a “two-toned color” but discolor in a particular pattern, scans should be taken that represent the entire surface area of the samples and the values averaged.
Avoiding pillowing
When collecting data, gently place the port on the sample applying just enough pressure to make sure that no light enters or exits the aperture. With too much pressure, the meat will form a curved surface (pillowing) that alters the reflectance compared with the desired flat meat surface. Generally, the weight of the instrument is sufficient to block light loss without pillowing the sample surface. When using small, hand-held instruments or hoods, let these rest on the meat surface, allowing their own weight to create uniform pressure. Any pressure applied by the operator(s) can be variable, thus affecting color readings.
Calculating myoglobin redox forms
In calculating the percentages of one or more of the myoglobin redox states, pay meticulous attention to details for the methods selected in Appendix C. Additionally, this type of data should be collected using an appropriate narrow spectral bandwidth spectrophotometer that provides the necessary wavelength precision to enable these measurements.
Downloading data
After collecting instrumental color data, researchers should download from the instrument to a computer or an appropriate app and save both tristimulus values and spectral data (if applicable). With spectral data, other calculations can be made in addition to those originally intended. Furthermore, spectral data can be converted from one illuminant to another. If using algorithms to calculate chroma (saturation index), hue angle, or K/S values (see Appendix E for details), verify that the values and decimal points are correct as described later.
Ratios for characterizing color
Ratios or differences of reflectance at selected wavelengths (see Figures 16–17 and Appendix C) and calculated color traits like chroma (saturation index) = (a*2 + b*2)1/2 and hue angle = [arctangent (b*/a*)] are commonly used to evaluate meat color (MacDougall, 1982). A description of various calculated parameters is available in Table 3.
Details on the calculation of various color parameters
| Color parameter | Purpose of calculation |
|---|---|
| 630 nm ÷ 580 nm or 630 nm − 580 nm | Larger ratios and differences indicate more redness due to either OMb or DMb; a ratio of 1.0 would indicate essentially 100% MMb (Strange et al., 1974) and a brown, well-done color in cooked meat (Tappel, 1957; Ledward, 1971; Howe et al., 1982; Lyon et al., 1986; Trout, 1989; Marksberry, 1992). This parameter has been used to follow color change during display, but it is not specific to OMb because DMb is also more red than MMb at 630 nm (Figure 8.2). |
| 650 nm ÷ 570 nm | Ratio values of ≈1.1 = no cured color; ≈1.6 = moderate fade; ≈1.7 to 2.0 = noticeable cured color; ≈2.2 to 2.6 = excellent cured color (Hunt and Kropf, unpublished data; see Figure 8.1). |
| 570 nm ÷ 650 nm | Ratios for cured meat successfully used by Barton (1967a, 1967b) where small values indicate less fade. |
| 537 nm ÷ 553 nm | Ratio to establish nicotinamide hemochrome as a pink color defect in uncured cooked light poultry meat. Higher ratios equal more nicotinamide hemochrome (Schwarz et al., 1998) (see Figure 8.2). |
| 474 nm ÷ 525 nm | Isosbestic wavelengths of OMb and MMb for calculating DMb |
| 572 nm ÷ 525 nm | Isosbestic wavelengths of DMb and OMb for calculating MMb |
| 610 nm ÷ 525 nm | Isosbestic wavelengths of DMb and MMb for calculating OMb |
| a* ÷ b* or b* ÷ a* | Larger ratios of a*/b* (or decreases in b*/a*) indicate more redness and less discoloration (Setser, 1984). |
| Chroma | C = (a*2 + b*2)0.5, with larger values indicating more saturation of the (saturation index) principle hue of the sample. Very useful to indicate intensity of whatever the hue is on the product. |
| Hue angle | Hue angle = [arctangent (b*/a*)]. Check your math carefully for HA. Larger values indicate less red, more MMb, and a more well-done cooked color (Bernofsky et al., 1959; Howe et al., 1982). HA is useful to indicate shifts in color over time toward discoloration. |
| Delta E | Total color change over a selected period of time. Generally calculated as ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]0.5. Very useful parameter to show total color differences over time. Various periods of time can be selected and compared. |
DMb = deoxymyoglobin; MMb = metmyoglobin; OMb = oxymyoglobin.
Objective measures of surface and subsurface pigments
For some experiments, objective visual methods—such as measuring the proportion of the surface area that is discolored with a grid, planimeter, or image analysis software—may be useful. Other studies might benefit from measuring the depth of myoglobin pigments from the surface using a digital caliper capable of discerning at minimum 0.1 mm. Still another method to consider is using near-infrared (NIR) tissue oximetry to calculate absolute amounts of surface and subsurface DMb, OMb, and MMb in samples using the methodology described by Mohan et al. (2010a).
Pitfalls of instrumental color measurement
Collection of both tristimulus and reflectance data
When collecting tristimulus data, researchers must understand fully the instrumentation and data software. Many software packages allow the tristimulus data to be converted to different illuminants. For example, the instrument may be set to record data using Illuminant A, but the software will allow these data to be converted to C and D65 later if spectra data from 400 to 700 nm are recorded and downloaded with the original tristimulus data. This function should be used when data from other illuminants might be of interest. To complete this data conversion, the instrument must be set to record spectral reflectance data in addition to tristimulus data to enable calculation of tristimulus values from other illuminants. Failure to collect spectral data will prohibit this conversion. Furthermore, spectral reflectance in the visible spectrum is necessary to estimate the percentage of myoglobin forms present on the meat surface or to use ratios that track fresh meat color change such as 630 nm ÷ 580 nm (redness indicator), 650 nm ÷ 570 nm for track cured meat color fade, and pinking defect 537 nm ÷ 553 nm (Figures 17–18).
Reflectance wavelength ratios useful for following redness (raw meat), cured pigment fading (cured, heat processed), and the pinking defect in poultry (uncured, cooked). Courtesy of M. C. Hunt and D. H. Kropf, Kansas State University. DMb, Deoxymyoglobin; MMb, Metmyoglobin; OMb, Oxymyoglobin.
Scanning modified atmosphere packages
Recent developments in fresh meat packaging technology have created unique problems with instrumental-reflectance measurements of meat samples. The proliferation of MAP with a gaseous headspace between the meat surface and the film has increased the difficulty of obtaining measurements during display. With traditional polyvinyl chloride–covered samples on foam trays, even repeated scanning of a sample throughout display is easy. With MAP, this becomes more cumbersome. The package must be inverted to allow the meat surface to contact the film surface to scan the meat’s surface. The package is then returned to its normal position with the meat no longer in contact with the film. Often, this maneuver results in the accumulation of moisture and/or fat smear on the film surface. Opening the package would compromise the modified atmosphere within the package and terminate the sample from the study.
Two techniques help minimize this potential problem. Some researchers prepare multiple sub-samples from the same original sample and package each sub-sample individually. All packages are displayed, and a single package is opened at predetermined intervals during the study to scan the meat surface. Although not a true repeated measurement, this prevents any interference that may occur from inverting packages during display. Deficiencies of this approach include package atmosphere variability and inherent differences among samples. The latter should be minimized when samples originate from the same original sample. If researchers choose this option, they must test the gaseous atmosphere of each package prior to opening to ensure that the target atmosphere has been maintained throughout display. The second option is to scan the same sample repeatedly during display by inverting the package but to do scans less frequently (not daily) to minimize package inversion. This offers the researcher a true repeated measure of the sample during display with minimal package variability and smear on the film surface. However, scanning less frequently (at these predetermined times during display) could result in not capturing the exact timing of color changes.
In many experiments, it may be useful to follow the changes of pigment redox forms below the surface of meat. Mohan et al. (2010a) used NIR tissue oximetry to measure both surface and subsurface quantities of myoglobin redox forms, which may provide new ways to analyze dynamic changes in color stability.
Nuances for calculating hue angle
Values for hue angle should be between 0° and 360°. Care must be taken for accurate calculations to get hue values to fall into the appropriate quadrant. Normally, meat products have positive values for a* and b*, and the hue values will be from 0° to 90° in the upper right quadrant of the CIE Color Space (Figure 10) for hue angle. For example:
It is critical to note that, if researchers encounter combinations of a* and b* with at least one or both of the values being negative, then comments by McLellan et al. (1995) become applicable. Refer to “Hue Angle” in Appendix E.
Nuances for calculating K/S values
Calculating the absorption and scattering coefficients (K and S) for determining the percentage of surface myoglobin redox forms requires care. When R (percentage reflectance) is put into the formula, K/S for a specific wavelength = (1 − R)2 ÷ (2R), the R must be expressed as a decimal, not as a percentage. When interpolation of the reflectance at wavelengths not given by the instrument (such as 474 nm, 572 nm) is needed, first calculate the reflectance at these wavelengths, and then convert to K/S values. Incorrectly entering information would mean that K/S values are calculated incorrectly by software and statistical programs and thus are valueless (Appendix C).
Notes on what measurements to include
It is notable that not all ratios or indexes must be calculated for all experiments. Investigators should select the ones most pertinent for the objectives of the study. Ideally, L*, a*, and b* data will correlate nicely with each other, other instrumental indicators of color, and visual observations. However, basing estimates of treatment effects on just one parameter (such as a* alone) may not tell the complete color history. Likely b* also reflects important color changes. Researchers should always collect a variety of data (especially both a* and b* and their calculated parameters) and then make informed decisions about which variables best depict color changes across and within treatments. All of these ratios and indexes need not be reported, but missing data cannot be reported at all, thus affecting reliability of results.
Reporting of instrumental details
In an article or report including instrumental color data, include the following information:
- i.
Instrument brand and model number
- ii.
Illuminant
- iii.
Aperture size
- iv.
Degree of observer
- v.
Standardization methods
- vi.
Data collected, tristimulus values, specified wavelengths, range of nanometers scanned, and any special parameters or calculations
- vii.
Number of scans per sample and whether the scans were averaged for statistical analysis
- viii.
Scanning frequency and whether a single sample was scanned repeatedly during display or whether different samples represented the same experimental unit
- ix.
Type of packaging used
- x.
If packages were opened or unopened at the time of scanning
Laboratory Procedures for Studying Myoglobin and Meat Color
Laboratory analyses, such as muscle pH and myoglobin concentration, help characterize color chemistry in skeletal muscle. Some additional reading of the literature will be necessary to properly apply and interpret data from these procedures, but these guidelines will be helpful in getting started with these procedures. Myoglobin chemistry is discussed earlier in these guidelines, but other reviews and book chapters that interrelate meat color and myoglobin chemistry will enhance understanding of the protocols detailed in these guidelines.
Fresh meat studies
pH
Meat pH is possibly the single most important factor affecting the color of fresh, processed, and cooked meat. Product pH should be reported in all meat color studies. Myoglobin oxidation (and browning) is significantly inhibited as pH increases from 5.6 to 8.5, whereas pH of 5.5 and below favors oxidation (Shikama and Sugawara, 1978; Yin and Faustman, 1993). Meat pigment solubility is also greatly affected by meat pH. Thus, extraction solutions are buffered to pH 6.8 for maximum yield of myoglobin and hemoglobin from meat samples (Warriss, 1979). Moreover, in preparing purified myoglobin, buffer solutions at pH 8.0 to 8.5 are used during centrifugation and dialysis steps, to minimize myoglobin oxidation (Faustman and Phillips, 2001). Myoglobin denaturation during cooking is also significantly lower at pH > 6.0 (Trout, 1989), accounting for the persistent pinking (hard-to-cook phenomenon) of ground beef patties made from high-pH, dark-cutting beef (Moiseev and Cornforth, 1999). Conversely, as pH decreases to 5.5–5.7, premature browning of beef patties during cooking will increase because lower pH favors formation of MMb (Hunt et al., 1999).
Measuring muscle pH accurately is difficult and must be done carefully. Spear-type muscle pH probes are available and can be used when properly calibrated. Many times, experimental conditions dictate that muscle probes be used. However, muscle probes are prone to technical error and poor repeatability. Thus, it is recommended that muscle pH be measured on muscle homogenates whenever possible. Protocol A in Appendix D is recommended for measuring pH of pre-rigor meat, using iodoacetate to inhibit glycolysis and prevent further production of lactic acid. Protocol B in Appendix D is recommended for post-rigor muscle or cooked meat products. Increasingly, investigators also measure the pH of individual muscles with a portable instrument equipped with a penetrating pH probe. As with all pH measurements, the device must be calibrated according to manufacturer instructions, using standard solutions for the pH range of interest (usually 4.0 to 7.0). Calibration solutions should be at or near the actual sample temperature.
Total fresh meat pigments
Meat pigment content is of interest because of its relationship to color intensity and, from a nutrition standpoint, as an indicator of heme iron content. Meat is an important dietary source of heme iron, which has a greater bioavailability. However, great total heme content may also initiate oxidative changes. Therefore, quantifying heme versus nonheme iron content in meat may be necessary (Carpenter and Clark, 1995).
Pigment extraction and spectrophotometry (transmission or absorption) are the methods of choice for total myoglobin and hemoglobin concentration. Protocol C in Appendix D describes a method for meat pigment extraction in cold phosphate buffer at pH 6.8 (Warriss, 1979). All pigments are converted to the reduced, deoxygenated form by adding sodium dithionite (Hunt et al., 1999). Pigment concentration is determined by absorbance of the deoxygenated pigments at 433 nm (the Soret peak). Protocol D in Appendix D describes a similar method for extracting meat pigments in cold phosphate buffer, but total pigment concentration is determined by absorbance at 525 nm, the isosbestic point for the 3 forms of myoglobin. Protocol D is based on the method of Krzywicki (1979) as modified by Trout (1989), and further modified by Tang et al. (2004). To measure the relative proportion of myoglobin forms the newer method by Tang et al. (2004) is recommended. To measure total pigment concentration in solutions, however, the methods provide equivalent results. The Trout (1989) and Tang et al. (2004) equations for total myoglobin differ only in using slightly different values for myoglobin molecular weight.
Total soluble meat pigments may also be measured using the classic method of Drabkin (1950). Pigments are extracted (Warriss, 1979), using 0.04 M phosphate buffer at pH 6.8 to maximize the amount of pigment extracted from normal-pH meat or using a buffer lower than 6.8 pH to prevent turbid pigment extracts from high-pH meat (de Duve, 1948; Hunt and Hedrick, 1977). Potassium ferricyanide and potassium cyanide are then added to a portion of the extract to convert pigments to the cyanometmyoglobin form. The concentration of myoglobin can then be determined spectrophotometrically, using the cyano-MMb absorption coefficient of 11.3 mM−1 cm−1 at 540 nm and myoglobin molecular weight of 17,000 (Drabkin, 1950).
Total heme pigment content of all meats (fresh, cooked, or cured) may alternatively be determined by extracting the heme group into acidified acetone, forming hemin (ferriprotoporphyrin chloride; Hornsey, 1956), as described in Protocols E and F in Appendix D. Karlsson and Lundstrom (1991) used more benign reagents (sodium hydroxide and Triton X-100) to extract heme as alkaline hematin (ferriprotoporphyrin hydroxide). Concentration of myoglobin was determined based on sample absorbance at 575 nm, in comparison to an alkaline hematin standard curve.
Separating myoglobin and hemoglobin
Crude extracts from skeletal muscles are sometimes used for myoglobin studies. However, to minimize the influence of other soluble sarcoplasmic proteins and enzymes, often using 100% pure myoglobin is necessary. In Protocol G of Appendix D, Faustman and Phillips (2001) detail a method for extracting heme pigments from muscle tissue through ammonium sulfate precipitation, followed by separation of myoglobin from hemoglobin using gel-filtration chromatography. This method can be used to purify myoglobin from various animal species, with only minor modifications in the initial level of ammonium sulfate precipitation. Myoglobin and hemoglobin concentrations can be determined by measuring the protein content of the purified fractions and considering initial sample weight and dilution factors.
High-performance liquid chromatography (HPLC) methods may also be used to quantify total heme pigment and the partitioning of myoglobin and hemoglobin (Oellingrath et al., 1990). Trout and Gutzke (1996) described an HPLC method to isolate myoglobin and determine its purity by calculating the area under the curves at 280 nm to determine myoglobin as a percentage of total protein and at 525 nm to determine myoglobin as a percentage of heme protein.
Relative proportion of myoglobin forms
The relative proportion of myoglobin forms (DMb, OMb, MMb, and COMb) at meat surfaces greatly affects color and retail acceptability. For example, when the proportion of MMb at the surface of retail beef products exceeds 40%, consumer acceptability drops significantly (Greene et al., 1971). The relative proportion of myoglobin forms at meat surfaces is measured by reflectance methods, as described in Appendix C(D). The proportion of myoglobin forms of muscle samples in solution after homogenization is measured by absorbance of their respective peaks (Trout, 1989; Tang et al., 2004).
Extraction techniques seldom prevent the conversion of one myoglobin form to another and provide no reliable information on redox stability in solutions. Krzywicki (1982) used low temperatures and controlled pH with buffers during extraction to minimize changes in MMb. Even so, some change occurred in the ratio of OMb to DMb. Krzywicki’s equations (Krzywicki, 1982) are widely used to estimate the relative proportion of different redox forms of myoglobin in solutions. Occasionally, these equations generate negative values for some redox forms, and sometimes the total estimates obtained by summation of the 3 redox forms exceed 100%. This was mainly attributed to selecting inappropriate wavelengths (545, 565, and 572 nm) in these equations. To solve this, Tang et al. (2004) used wavelength maxima at 503 nm for MMb, 557 for DMb, and 582 for OMb. The revised equations performed better relative to negative values and summation to 100% of the redox forms (Table 3).
Differentiating carboxymyoglobin and oxymyoglobin in solution
The absorbance spectra of the 2 cherry red–colored redox forms, COMb and OMb, are very similar (Figure 19). Traditional equations used to estimate myoglobin redox forms (Krzywicki, 1982; Tang et al., 2004) do not account for the presence of COMb. Using these equations to determine brown pigment (MMb) formation in pure solution of COMb provides erroneous results such as negative values and sums exceeding 100%. This has been solved using the ratio A503/A581 as a browning index, which represents an indirect estimate of MMb formation (Suman et al., 2006). The usefulness of the browning index was verified using combinations of COMb, OMb, and MMb in split cuvettes.
Absorption spectra of MMb, COMb, and OMb solutions containing equivalent myoglobin concentrations. The arrows indicate the isosbestic point at 525 nm, MMb absorption peak at 503 nm, COMb absorption peak at 543 nm, and OMb absorption peak at 582 nm. Courtesy of Dr. S. P. Suman, University of Kentucky, and Dr. C. Faustman and Dr. R. A. Mancini, University of Connecticut. COMb, carboxymyoglobin; MMb, metmyoglobin; OMb, oxymyoglobin.
Nam and Ahn (2002) reported β and α peaks of OMb at 541 and 576 nm in the drip from aerobically packaged turkey breast, with a shift to shorter wavelengths (536 and 566 nm, respectively) after irradiation. Reflectance spectra were also used to differentiate OMb and COMb. Gas chromatography verified production of CO in irradiated samples. Thus, COMb was the source of the pink pigment of irradiated turkey breast muscle (Nam and Ahn, 2002).
The β and α peaks of horse OMb are 544 and 582 nm, respectively (Bowen, 1949), with a slight shift to shorter wavelengths (540 or 541 nm and 577 nm) for COMb (Bowen, 1949; Sørheim et al., 2006). Although it is theoretically possible to differentiate COMb and OMb based on their characteristic spectra from 400 to 700 nm, it is not currently possible to determine their relative proportions in meat samples exposed to both CO and O2.
Mitochondrial oxygen consumption
Mitochondrial activity plays a major role in postmortem muscle oxygen consumption, affecting rate of myoglobin oxygenation and color stability. As postmortem age of muscles increases, mitochondrial activity tends to decrease. High storage temperature and high pH greatly influence postmortem mitochondrial activity (Cheah and Cheah, 1971; Ashmore et al., 1972; Bendall and Taylor, 1972; Cornforth and Egbert, 1985). Oxygen consumed by meat affects myoglobin oxygenation because of competition for available oxygen between mitochondrial enzymes and myoglobin. With a decrease in mitochondrial activity postmortem, myoglobin oxygenation occurs at a higher rate. In meat, many cellular processes and organelles compete for available oxygen, affecting myoglobin redox stability and MMb reduction postmortem (Ramanathan et al., 2009). Protocol H in Appendix D describes methods for isolating mitochondria, and Protocol I in Appendix D describes methods for measuring mitochondrial OCR.
In addition, mitochondrial content of skeletal muscles differs by physiological origin, causing differences in relative OCR, myoglobin redox forms on surface and at subsurface levels, and meat color stability. The ability of fresh meat to retain a bright cherry red color of “bloomed” meat during storage and display differs among muscles (Atkinson and Follett, 1973; Hood, 1980; O’Keeffe and Hood, 1982; Renerre and Labas, 1987; Mancini and Hunt, 2005). Muscles with higher mitochondrial content tend to have a higher OCR and form more MMb. Likewise, muscles with a high discoloration rate tend to have low color stability and high OCR (O’Keeffe and Hood, 1982; Renerre and Labas, 1987). Atkinson and Follett (1973) also noted that higher OCR in skeletal muscles was associated with higher rates of discoloration. Measurement of OCR can help determine mitochondrial activity of postmortem skeletal muscles of different physiological origin and their relative color stability (Protocols I and J of Appendix D).
Meat scientists have developed objective procedures to determine muscle oxygen uptake and OCR, among them the Warburg flask (Urbin and Wilson, 1961), differential respirometry (DeVore and Solberg, 1975), Clark oxygen electrodes (Lanari and Cassens, 1991; Ramanathan et al., 2009), reflectance spectroscopy (Madhavi and Carpenter, 1993), and headspace oxygen analyzers (Sammel et al., 2002a). Interactions between light and meat pigments offer an opportunity to develop methodology for detecting the redox dynamics of myoglobin using NIR (700 to 1,000 nm) technology. Mohan et al. (2010a) used a frequency-domain multi-distance NIR tissue oximetry that provides a real-time, noninvasive, and direct measure of myoglobin oxygen saturation and OCR in skeletal muscle.
Metmyoglobin reducing capacity
The capacity of muscle to reduce MMb is a large contributor to color stability. This capacity has been estimated using a measure of enzyme activity in a muscle homogenate with an excess of NADH as well as the ability of the muscle to reduce after being exposed to a mild oxidant. Often, the term MMb reducing activity is used for both methods. However, the relationship between these measures and color change is quite different. Thus, readers must be quite careful in interpreting the existing literature.
Methodology used to determine MRA of meat differs widely among investigators (see the review by Bekhit and Faustman, 2005). The most common procedure for determining MRA starts by inducing high initial levels of MMb (usually by packaging in 1% O2 atmospheres or NO2−-induced oxidation) followed by an assay step that promotes MMb reduction. Changes in total MMb content during this reduction step are used to estimate the muscle’s reducing ability. However, meat color researchers often question the validity of the most appropriate means of presenting and interpreting this change in MMb levels.
Mancini et al. (2008) assessed location effects (surface and subsurface) on MRA following display and evaluated the influence of package oxygen concentration on location effects and MRA. They also examined the relationship between MMb reduction measurements (initial MMb formation vs. post-reduction MMb vs. absolute amount reduced vs. relative amount reduced) and color stability. Their study demonstrated a positive correlation among the 4 MMb/MRA measurements and visible surface color stability data. These investigators reported that, regardless of muscle, subsurface reducing activity measurements did not correlate with surface color stability. They found that, for all muscles used in their study, traditional absolute and relative MRA calculations measured on the steak surface correlated least with surface color.
Faustman and Cassens (1990) reported both absolute and relative aerobic reducing activity. O’Keeffe and Hood (1982) proposed that relative MRA was less accurate in predicting muscle color than absolute MRA owing to differences among muscles to form MMb. McKenna et al. (2005) reported that some muscles resisted development of surface MMb when samples were placed in a 1% O2 environment. They used resistance to induced MMb formation to relate muscle-reducing capacity to color stability. Sammel et al. (2002a) reported that NO-MRA was useful for measuring reducing activity. These researchers suggested that because their method initially used a mild oxidant (NaNO2), it may offer a more practical approach for determining MRA than assays that use ferricyanide. King et al. (2011) used the method of Sammel et al. (2002a,b) to monitor animal-to-animal variation in the color stability of beef longissimus steaks. They reported that initial steak OCR, initial MRA after NO2− treatment, and post-reduction MMb levels were important in determining the color stability of individual steaks.
Protocol J in Appendix D describes an assay for MRA of intact muscle slices, adapted from the method of Watts et al. (1966). Muscle pigments at the sample surface are initially oxidized to MMb by a soaking of the sample in a sodium NO2− solution for 20 min. The slice (1.27 cm thick) is vacuum packaged, and surface percentage MMb is monitored for 2 h at 30°C by a measurement of reflectance K/S ratios (572/525 nm). The sample reducing ability is defined as the percentage decrease in surface MMb concentration during the incubation period. Protocol K of Appendix D describes modifications to Protocol J for measuring MRA of minced meat samples (Sammel et al., 2002a,b).
Protocol L of Appendix D describes a rapid (2 min) assay for MMb reductase activity measurement in muscle homogenates (Hagler et al., 1979; as modified by Madhavi and Carpenter, 1993). The reaction is initiated by adding muscle filtrate + NADH to a solution of MMb + ferrocyanide in a spectrophotometer cuvette. MMb reductase activity is monitored by the increase in absorbance of OMb at 580 nm during the initial linear phase of the reaction (1 to 2 min).
Effects of added substrates on MRA (lactate, malate)
Several researchers have become interested in the potential for generating NADH through endogenous enzymatic systems that facilitate MMb reduction, including biochemical processes that could potentially contribute to meat color stability. Watts et al. (1966) demonstrated that adding nicotinamide adenine dinucleotide (NAD+) increased MRA in meat. Electron transfer can also generate NADH from added substrates, such as succinate or cytochrome c, to NAD+. With appropriate substrates, several dehydrogenases in the cytoplasm can generate NADH (Bodwell et al., 1965). Giddings and Hultin (1974) and Giddings (1977) suggested that mitochondria or sub-mitochondrial particles could help reduce MMb and hypothesized that mitochondria are involved in MMb reduction by supplying the meat tissue with key reducing cofactor–reduced NADH, generated by endogenous enzymes or by reversing electron transport. Recently, Kim et al. (2006) and Mohan et al. (2010b) demonstrated the effects of added substrates from glycolysis (lactate) or mitochondrial tricarboxylic acid pathways (malate). Both studies reported improved MRA in meats after substrates were added to intact muscles or to skeletal muscle homogenates.
Cooked meat studies
Color and color uniformity are important criteria for retail acceptance of cooked meats. Cooked meats are generally gray-brown because of the formation of globin hemichromes via fresh meat pigment denaturation, coagulation, and oxidation during aerobic heating. However, cooked meats may also be red, pink, or prematurely brown, depending on a variety of factors, including cooking temperature/time, meat pH, anaerobic (reducing) conditions, the presence of various pinking compounds (including CO or NO gases), and unintentional contamination with NO2− or NO3− salts.
Meat pigments (myoglobin, hemoglobin) denature during cooking, causing unfolding of globin. Under aerobic conditions, the heme iron is readily oxidized, and the exposed heme may form complexes with denatured proteins, including dimers or aggregates of apomyoglobin (Tappel, 1957; Ledward, 1971). The resulting gray-brown complexes are termed denatured globin hemichromes, with “hemi” denoting the oxidized state of the heme iron. Although visible spectrum absorption has been used on myoglobin solutions during heating, reflectance spectroscopy is typically used to study cooked meat pigments (Ledward, 1971). Protocol M in Appendix E describes a reflectance method for detecting the pink denatured globin hemochromes of anaerobic-cooked meats (>76°C). Care must be taken to conduct any analysis in a timely manner because these pigments fade rapidly in air (Ghorpade and Cornforth, 1993; Cornforth, 2001).
Persistent pinking and premature browning—Diagnostic methods
Consumers are sometimes sensitive to the pink color of cooked meats, suspecting that the product may be undercooked. Thus, processors occasionally need to test their products to identify the cause of—and eliminate or minimize—unwanted pinking. Nitrite or NO3− contamination of ingredients is one possible source of unwanted pinking (Heaton et al., 2000). This possibility may be examined using the Hornsey (1956) test for cured meat pigment (Protocols E and F). Surface pinking of grilled meats also results in a positive test for cured meat pigment because of exposure to nitrogen dioxide in combustion gases (Cornforth et al., 1998). Combustion gas may contain CO, but the Hornsey (1956) method is specific for NO-heme and does not detect CO-heme groups.
If the meat is indeed undercooked, undenatured myoglobin will be present at higher-than-normal levels. Myoglobin is resistant to heat denaturation at pH of > 6.0, resulting in higher-than-normal myoglobin concentration in cooked meats and red or pink interior color at internal temperatures of 80°C or higher (Trout, 1989; Moiseev and Cornforth, 1999). At the other extreme, Hague et al. (1994), Lavelle et al. (1995), and Hunt et al. (1999) described premature browning in oxidized ground beef, in which MMb denatured at a lower temperature than OMb or DMb during cooking. Premature browning of hamburger has food safety implications because patties may be seen as fully cooked at a cooking temperature insufficient to kill foodborne pathogens.
Soluble myoglobin can be extracted in phosphate buffer and measured as described in Protocol D, using a spectrophotometer to measure absorbance at 525 nm, the myoglobin isosbestic point. Some soluble myoglobin may remain in cooked samples, depending upon species and internal cook temperature. Meat with pH > 6.0 will have higher-than-normal levels of soluble myoglobin. On the other hand, prematurely browned meats have lower-than-normal levels of soluble myoglobin, possibly associated with low pH.
If pinking due to undenatured myoglobin or cured meat pigment is not confirmed, the presence of denatured globin hemochromes should be suspected. These pink pigments are formed under anaerobic, high-heat conditions, such as canning or Crock-Pot cooking while submerged under water. Presence of these pigments may be confirmed by Protocol M.
Cured meat studies
These guidelines detail laboratory methods for detecting and quantifying cooked and cured meat pigments and precursor compounds. Cured meats are typically formulated with sodium or potassium NO2 or NO3, forming the pink cured meat pigment (mono-nitrosyl-hemochrome) during cooking. In naturally cured meats, the curing agent is usually NO3− as a component of celery seed powder. In traditional fire-dried jerky or BBQ meats, NO2 gas formed during combustion is a potent pinking agent, owing to its ability to react with water to form NO2− ions on moist meat surfaces, causing surface pinking (curing) during cooking.
Cured meat pigment
Erdman and Watts (1957) developed an effective method for following changes in cured meat color by monitoring the surface reflectance ratio of wavelengths 570 nm/650 nm. This measure is useful to indicate leaky vacuum packages or other conditions that promote color fading. The cured meat pigment was identified as mono-nitrosyl-hemochrome by Killday et al. (1988). Those authors used mass spectroscopy to demonstrate a mass increase of 30 for the nitrosylated heme, corresponding to binding of 1 NO group (not 2, as previously reported). Cured meat pigment content, as a percentage of total heme pigments, is a useful measure of the effectiveness of the curing process (Hornsey, 1956). The hemochrome itself is not soluble because it is a complex with heat-denatured proteins. However, the NO-heme group may be extracted in 80% acetone (adjusted for the water content of the sample) and quantified by spectroscopy at 540 nm (Protocols E and F in Appendix D).
Total heme and heme iron content
Total heme content can be determined by extracting all heme groups into acidified 80% acetone, including cured and uncured pigments as well as heme-containing enzymes and cofactors (Protocols E and F). Total heme content (as hematin) is measured by absorbance at 640 nm (Hornsey, 1956). Less than 80% of the heme pigments converted to the nitrosylheme form is generally considered acceptable pigment conversion during curing (Pearson and Tauber, 1984). In the Hornsey (1956) procedure, 10-g samples were mixed in tall beakers to prevent undue evaporation. Pearson and Tauber (1984) used 2-g samples and capped test tubes to prevent evaporation and allow analysis of more samples at a time. Carpenter and Clark (1995) used 5-g samples.
Total heme measurement using the Hornsey (1956) method can assess the nutritional values of heme iron content of meats (Carpenter and Clark, 1995). Total iron content of meat samples after wet ashing can be determined by the ferrozine assay (Carter, 1971), in which binding of Fe2+ to ferrozine forms a red pigment measured by spectroscopy at 562 nm. Nonheme iron content can be determined using ferrozine to detect iron in HCl-trichloroacetic acid extracts (Schricker et al., 1982). Stainless steel probe-type homogenizers should not be used to homogenize samples in HCl-trichloroacetic acid, because iron will be extracted from the probe itself, particularly older, worn probes (Jayasingh, 2004).
Red pigment of Parma ham
Parma ham is a traditional fermented meat product of Parma, Italy, prepared by lengthy seasoning of pork legs, without adding NO3− or NO2− curing salts. Morita et al. (1996) used electron spin resonance spectroscopy to show that the red pigment of Parma ham differed from NO2−-cured meat pigment. They further demonstrated that staphylococci isolated from Parma ham generated a red myoglobin derivative from MMb. Wakamatsu et al. (2004) characterized the bright red pigment of Parma ham spectroscopically and fluoroscopically and used HPLC and electro-spray ionization high-resolution mass spectroscopy. They found that the red color was caused by Zn–protoporphyrin IX, not iron-based heme pigments.
Nitrite in ingredients and residual nitrite in meat
Protocol N in Appendix E describes a method for determining the NO2− content of ingredients or residual NO2− level of cured meats after cooking and during storage based on reactivity of NO2− with N-1-naphthyl-ethylenediamine-2-HCl and sulfanilamide; this forms a red complex with maximum absorbance at 540 nm (AOAC, 1990). Ingredient or product NO3− levels can be measured after sample extracts are treated with cadmium (Sen and Donaldson, 1978; Sen and Lee, 1979), which reduces NO3− to NO2−. Nitrite analysis, as described in Heaton et al. (2000) can then be done. NO3− = total NO2− −initial NO2− (AOAC, 1990). More recently, vanadium has replaced cadmium as a NO3− reducing agent because of environmental safety concerns (Miranda et al., 2001; Doane and Horwáth, 2003). The NO3− assay using vanadium is described in Protocol O of Appendix D.
Gaseous components from gas combustion ovens
The concentration of gaseous precursors to pink pigments (NO2, CO, NO) in combustion ovens, for example, can be determined using a chemiluminescent gas analyzer (Cornforth et al., 1998). To measure NO, the gas sample was blended with ozone (O3) in a flow reactor, in which NO + O3 → NO2 + O2 + hv. Light emission occurs when the excited NO2 molecules decay to lower energy levels, measured by spectroscopy. To measure NOX (NO + NO2), the sample gas is first diverted through an NO2 to NO catalytic converter. Nitric oxide is then measured as previously described. Oxygen can be measured with a paramagnetic oxygen measurement system. Paramagnetic oxygen can become a temporary magnet when placed in a magnetic field. Most other gases are diamagnetic and therefore unaffected. The instrument measures the magnetic susceptibility of oxygen in the gas sample.
Carbon monoxide can be measured with a non-dispersive infrared analyzer. The instrument produces infrared radiation from 2 separate energy sources. Radiation is modulated by a chopper into 5-Hz pulses, which pass through optical filters to reduce background interference from other infrared-absorbing components. Each infrared beam passes through a separate cell, one of which is sealed and contains the reference gas (CO). The other cell contains the continuously flowing sample gas. The quantity of infrared radiation absorbed is proportional to the CO concentration.
Packaging measurements
Because the color of fresh and processed meat is so profoundly influenced by ligands bound to the heme moiety, and because packaging is used commercially to minimize fresh and processed meat color deterioration, packaging requires special consideration in laboratory analysis. The following are important considerations for packaging of samples during analysis.
Film thickness
Digital micrometers capable of measuring thicknesses in mils (1/1000 inch [see Appendix E]) are useful for measuring film and package tray thickness. Many vacuum package bags are 2 to 3 mils thick, whereas oxygen-permeable polyvinyl chloride film overwrap of fresh retail meats is often <1 mil thick. Generally, as film thickness increases, gas permeability decreases. Thicker films are also more expensive.
Film permeability
For fresh meat, high oxygen permeability films maintain oxy-heme pigments (Landrock and Wallace, 1955; Cornforth and Allen, 2009). Extremely low oxygen permeability films (also known as high barrier films) will encourage deoxyheme pigments to form because of the reducing capacity of the meat (Siegel, 2007). Conditions resulting in a low partial pressure of oxygen (1 to 25 mm Hg) will encourage rapid oxidation and pigment browning (Kropf, 2004), and the longer the time spent in this oxidizing state, the more difficult it becomes to reduce the MMb. Table 4 illustrates the change in atmospheric pressure and oxygen partial pressure at various levels of vacuum, from zero (vacuum) to 1 atmosphere (760 mm Hg). The danger zone for most rapid browning is highlighted. Using a typical vacuum packaging machine makes it very difficult to reduce oxygen levels below this range; thus, MMb will likely form until the meat has consumed (scavenged) the residual oxygen in the package. MAP packaging may reduce the residual oxygen levels more than vacuum packaging owing to 1 or multiple flushes of the desired atmosphere into the packaging chamber. With any packaging system, care must be taken to obtain sufficiently low partial pressure of oxygen so that the ability of the meat to consume the residual oxygen is not exceeded. Regardless of degree of vacuum pulled in the package, the residual atmosphere contains the same relative gas proportion as air, 78.1% nitrogen, 20.9% oxygen, 0.9% argon, and 0.03% carbon dioxide. However, the gas concentration detected by a sensor like an oxygen electrode is proportional to the atmospheric pressure around the product. For example, at a partial vacuum of 1/2 of atmospheric pressure, the measurable oxygen concentration oxygen is 10.45% (104,500 ppm) for an oxygen partial pressure of 79.6 mm Hg (380/760 × 159.2 mm Hg; Table 4). The permeability of the packaging film is also critical in keeping ingress of oxygen low, especially in products with a long shelf life, such as those commonly used for export.
Oxygen concentration and partial pressure at various degrees of vacuum packaging of fresh meat
| Level of vacuum (%) | 100 | 99.4 | 85.3 | 75.0 | 50.0 | 25.0 | 0 |
|---|---|---|---|---|---|---|---|
| Vacuum gauge (in Hg) | 29.92 | 28.42 | 25.43 | 22.44 | 14.96 | 7.48 | 0 |
| Total gas pressure in the package (Torr = mm Hg) | 0 | 38 | 114 | 190 | 380 | 570 | 760 |
| Oxygen concentration (%) | 0 | 1.1 | 3.14 | 5.23 | 10.45 | 15.67 | 20.9 |
| Oxygen concentration (ppm) | 0 | 11,000 | 31,400 | 52,225 | 104,500 | 156,700 | 209,000 |
| Partial pressure of oxygen in the package (mm Hg = Torr) | 0 | 1.05 | 23.9 | 39.8 | 79.6 | 119.4 | 159.2(Air) |
Bolded values are in the range of low-oxygen partial pressure (1 to 25 torr) and very low percentage oxygen of 1% to 3% that favors rapid formation of MMb. Extended time in this oxidative state can be damaging to future color stability. Vacuum and MAP will reduce the residual oxygen levels immediately after packaging, but without good practices, the shaded area will be reached, meaning that more oxygen than is desirable or normal remains. Multiple cycles with vacuum or multiple flushes with MAP may help achieve the desired package atmosphere with the lowest possible residual oxygen. The packaging machine vacuum gauge indicates excellent vacuumization; however, the critical measurement is the vacuum level actually occurring in the package (see Kennedy Gauge in Appendixes B and E). If post-packaging oxygen levels exceed 3%, then greater amounts of oxygen must be consumed by the muscle’s oxygen-scavenging enzymes to help minimize MMb formation. With anoxic packaging, OMb will form MMb, but the less time spent in this critical atmosphere, the greater the color stability. If the meat temperature is too cold for the color chemistry to operate, the meat color may suffer from extended exposure to oxidative conditions; warming the meat slightly will solve this problem for many muscles.
MAP, modified atmosphere packaging; MMb, metmyoglobin; OMb, oxymyoglobin.
Gas permeability properties of packaging films, bags, and trays usually include transmission rates for water vapor and oxygen transmission rate. Transmission values for carbon dioxide, CO, and nitrogen are less frequently available. Film permeability may be expressed per 100 in2 or 1 m2. The equation to convert gas permeability values per unit area (British or metric) is as follows: Gas permeability expressed in cc/100 in2 × 15.5 = Gas permeability of the film expressed in cc/meter2. Permeability values also vary with other film factors in addition to film thickness. Research reports should include information about film permeability.
For cured processed meats, the nitrosylheme moieties are very sensitive to combinations of light and oxygen, resulting in oxidation of cured pigments. Thus, residual oxygen of less than 0.15% initial O2 (Larsen et al., 2006) and films with extremely low oxygen permeability (<0.1 cc O2/100 in2/24 h, equivalent to <15.5 cc O2/m2/24 h) are generally used (Siegel, 2007).
Additionally, samples must be held under dark storage conditions for 24 to 96 h after packaging to permit self-scavenging of residual oxygen before they are exposed to light (Møeller et al., 2003). This is particularly important when evaluating product color stability under display lighting conditions.
Modified atmosphere packaging
High oxygen barrier packaging films can be used in combination with a variety of headspace gases to manipulate and preserve pigment forms in meat. Carbon dioxide is well known for its antimicrobial effect in MAP. However, nitrogen and carbon dioxide are essentially neutral in their effects on pigment forms and therefore their presence in a MAP headspace will not affect color (Møeller et al., 2004). High oxygen can help maintain oxy-heme pigment forms (Georgala and Davidson, 1970; O’Sullivan and Kerry, 2010), but respiratory capacity of the meat must be considered to avoid depleting oxygen to a level that promotes the formation of MMb (Bekhit and Faustman, 2005). Carbon monoxide or NO gases in the package headspace or use of packaging films impregnated with sodium NO2− crystals will result in pigment forms that reflect the binding of those compounds (Siegel, 2007, 2009).
Measurement of package gas composition
To show that MAP systems achieve desired gas composition and that the desired gas composition was maintained throughout storage, report the gas composition in packages. The relative gas compositions in MAP change dynamically during package shelf life because of meat respiration and because meat absorbs gases; thus, with gases permeating through the package film, accurately describing the time of measurement is important. Samples drawn from the packages with a syringe through self-sealing septa allow using headspace gas analyzers to measure oxygen, CO, and carbon dioxide concentrations (Knock et al., 2006; Mancini et al., 2009; Raines and Hunt, 2010).
Effect of lipid oxidation on meat color (fresh, cooked, cured)
Many meat color studies include measures of lipid oxidation, because myoglobin oxidation is often closely linked with lipid oxidation. Aldehyde products of lipid oxidation initiate conformational changes in myoglobin, causing increased heme oxidation and browning (Alderton et al., 2003). Hemin released from fish hemoglobin during storage also stimulates lipid oxidation (Grunwald and Richards, 2006). Similarly, ionic iron released from heme during heating may stimulate lipid oxidation, as measured by the assay for thiobarbituric acid reactive substances (TBARS; Igene et al., 1985). The extent of lipid oxidation can be measured using many techniques, including headspace analysis of volatile oxidation products (Watanabe et al., 2008), and sensory evaluation, but the TBARS test is most often used in meat products.
The TBARS test is based on the development of a pink chromagen with maximum absorbance at 530 to 535 nm upon reaction of thiobarbituric acid with aldehyde products of lipid oxidation, particularly 2,4-alka-dienals (Marcuse and Johansson, 1973). Malonaldehyde is the compound used for TBARS standard curves. TBARS test results may be obtained in 1 d or less for multiple samples, and TBARS values correlate well with sensory testing. TBARS values > 1.0 ppm are usually associated with detectable oxidized odor and flavor of cooked meat samples (Greene and Cumuze, 1981). Tarladgis et al. (1960) developed the widely used distillation method (Protocol R in Appendix D), in which 2-thiobarbituric acid solution was added to the sample condensate. To avoid the distillation step, 2-thiobarbituric acid solution may be added directly to the meat sample, allowing several hours for chromogen formation in unheated samples (Witte et al., 1970), or the sample can be boiled for 10 min, as described in Protocol Q (Buege and Aust, 1978).
A yellow chromogen with maximum absorbance at 453 nm also develops in the TBARS test in the presence of many lipid-derived aldehydes (Marcuse and Johansson, 1973) and sugars, including sucrose (Du and Bramlage, 1992). To correct for the yellow interference caused by sugars, Du and Bramlage (1992) developed a modified procedure using standard curves for both malonaldehyde and sucrose. Alternatively, yellow color development has been avoided in meat samples containing raisins (70% sugar) by using the original distillation method of Tarladgis et al. (1960) because sugar aldehydes are not volatile and not collected in the sample condensate (Vasavada and Cornforth, 2006).
In cured meats, TBARS values are affected by the presence of residual NO2−. Accordingly, the modified TBARS method of Zipser and Watts (1962) adds sulfanilamide to cured meat samples before distillation to prevent erroneous results caused by the nitrosation of malonaldehyde by the residual NO2−. However, adding sulfanilamide also affects the TBARS value. TBARS values of meats cured with 100 to 200 ppm NO2− were always higher when sulfanilamide was present than in its absence. However, at low levels of 0 to 50 ppm sodium NO2−, the TBARS values were always lower in the presence of sulfanilamide (Shahidi et al., 1985).
Fundamental research methods
Mass spectrometric characterization of myoglobin
Meat color stability depends on many intrinsic and extrinsic factors, among them species-specific variations, distribution of red and white muscle fibers, and myoglobin chemistry. Mass spectrometry is a key analytical tool particularly useful in the structural characterization of proteins. In meat science, this technique has shown great promise in the functional characterization of myoglobins. In an attempt to characterize the species-specific variations in meat color, Joseph et al. (2010a) used matrix-assisted laser desorption-ionization time-of-flight mass spectrometry to characterize myoglobins of bison, Joseph et al. (2010a) for turkey, and Suman et al. (2010) for emu.
Proteins and peptides are major constituents of muscle foods, vital to determining process-induced modifications in food proteins. Food proteomics has started to influence many aspects of the food chain, including food production, food safety, and quality assurance. The use of mass spectrometry in recent years has revolutionized protein characterization, amino acid sequencing, and fingerprinting of bacterial proteins. Recent advances in applying proteomics offer opportunities for meat scientists to explore the molecular basis of ingredient interactions for both meat color and color stability.
Mancini et al. (2010) used matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for determining the mechanisms by which lactate influences beef color stability. Using mass spectrometry allows systematic analysis of ingredient interactions, process-induced modifications, and identification of areas of the food chain that are the most vulnerable to quality defects, microbial contamination, and nutrient deterioration. Despite being relatively new to meat science, research in food proteomics has already helped improve human health.
Oximetrics to measure relative concentration of myoglobin forms in packaged meats
Biochemical factors that contribute to meat color have been an important area of research for decades, but little research has focused on the development of noninvasive methods and/or techniques for estimating the overall quality of meat rapidly, in real time. Although this area has seen significant progress, existing techniques to characterize meat color parameters and predict meat color stability are limited because they are invasive, time consuming, labor intensive, and provide only an indirect estimate of myoglobin redox status. Similar techniques to characterize tissue structure related to biochemical processes like oxygen consumption and mitochondrial activity suffer from the same limitations. Interactions between light and muscle pigment in meat offer an opportunity to develop methods for detecting the redox dynamics of myoglobin using NIR (700 to 1000 nm) technology.
Near-infrared spectroscopy (NIRS) has been used extensively to determine oxygen absorption by myoglobin and hemoglobin in medical diagnostics and exercise physiology (Ferreira et al., 2005). NIRS is noninvasive, continuous, and rapid (25 to 35 s) method for estimating the absolute concentration of oxygenated and deoxygenated myoglobin at surface and subsurface levels in meat (Mohan et al., 2010a).
A fundamental approach based on light–tissue interaction of NIRS could provide valuable information about optical properties and absorbance patterns of the meat in real time with quantitative information of myoglobin redox forms on meat surfaces and at subsurface levels. Because NIR light penetrates deeply into biological tissues like meat, NIRS could be a potentially effective technique for noninvasive, macroscopic imaging of postmortem muscle. Because myoglobin and hemoglobin absorb at the same wavelength in the NIR region, the same approach can be used to determine the redox stability of myoglobin and other structural features of meat that would eventually allow us to understand the effects of post-processing on myoglobin chemistry.
Application of “omics” technologies
Technologies to profile the genome, transcriptome, proteome, and metabolome have expanded exponentially. This has enabled meat scientists to examine the biological basis of meat quality phenotypes, including lean color and lean color stability. Discussion of the details of these technologies is beyond the scope of these guidelines. However, considerations should be made in utilizing these methods to study meat color.
Understanding the complexities of the phenotype and exactly what is being measured is essential for proper interpretation of these data. It is important to remember that, in these experiments, sample sizes are typically small and each sample essentially constitutes its own treatment. Thus, to control error, phenotyping must be accurate and repeatable. When possible, component traits, such as reducing ability and oxygen consumption, can greatly aid in understanding the mechanisms being revealed.
Experimental design is critical. These technologies are extremely powerful in identifying differences between groups of samples. Interpretation of the results depends on relating the compounds that are identified to treatment differences. Thus, it is essential that sampling be conducted in a manner that ensures that the differences of interest are the only differences that exist between treatment groups. Finally, identification of compounds remains a challenge for these technologies, particularly metabolomics. At this point, many results will include unknown compounds. It is tempting to focus only on compounds that can be annotated by existing databases. However, investigators are encouraged to report, or otherwise make associations with, unknown compounds so that as annotation becomes more complete these associations can be investigated further.
Photography of Meat
Documenting color and other meat package appearance traits in photographs is an important component of meat color research. However, red—the primary color of meat—is difficult to reproduce accurately in photographs. Therefore, capturing realistic images of red meats and their varying degrees of discoloration is challenging. Special equipment is needed for packaging, lighting, cameras, image processing, and transfer of images to print. Printing high-quality color photographs of meat is best done by professional photo printing companies and will not be addressed in these guidelines. However, to create images appropriate for printing, Color Separation and Gray Scale Guides are essential. During photo shoots, the first and last photos taken in a series should include these guides (see Figure 20 for photo with meat with color separations guide and Appendixes B and E for information about color separations guide and gray card). As with all photography, optimize the exposure and focus exactingly.
Kodak Color Separations Guide (or any equivalent guide) should be included with the photographed meat photo so that the picture can be adjusted to achieve the most accurate coloration possible. Inclusion of a Kodak Gray Scale Guide (or any equivalent guide) is also recommended for the photograph.
Packaging
Although the best color reproductions are obtained with unpackaged meat, meat packaged in glossy films can be photographed with care. For meat packaged in modified atmosphere packages, the effects of headspace and moisture under the top film must be considered. The use of anti-fog films for meat photography is recommended. Consider slightly tilting (without bumping) the package before the photo shoot to allow any condensate to run to the edge of the package. If packaged meat is to be photographed, white or black trays are recommended.
Lighting and background conditions
Photography of meat is best done in rooms completely shielded from daylight. Proper lighting is essential to successful food photography. Light sources can either be flashes, permanent lighting, or both and may be of tungsten, incandescent, high-intensity discharge (e.g., metal halide), halogen, or LED. Some fluorescent lighting should be avoided because of unfavorable light spectra and varying light intensity over time. The typical warm white light used for room and table lighting can give an undesirable yellow to green tinge to meat surfaces. The position of the light should be 45° to the meat from 2 opposite sides to minimize reflection on the surface.
If photography is performed with daylight, try to avoid direct sunlight on the objects. Be aware that the color temperature will change depending on weather and time of day. Blending daylight with in-room light sources is not recommended.
Semi-translucent, light-diffusing fabric between the light source and the meat can be useful to evenly distribute light and reduce shading or shadowing. Small, simple tents are available for this purpose.
For flash photography, a professional lighting system for even lighting is recommended. Although not always ideal, it is possible to adequately photograph meat with flashes directly attached to the camera. In this scenario, experiment with various flash positions to minimize gloss or glare on the fresh meat surface or packaging material. Covering the flash with 1 or 2 layers of white hair or beard netting or lens paper may help diffuse the light, resulting in a more uniform color with less light glare and glossiness.
When selecting the background for the meat or package, use a material with a different color than the subject. This will ease the photo editing process if the subject of the photo must be isolated. A 20%-black background (a light gray) is often a good solution for meat (isolated or packaged). White and stainless steel backgrounds can cause blurring of the object, under exposure, and blending with some packaging materials.
Camera and lens selection
The digital camera should have a resolution of at least 10 megapixels and should have a direct image output for data transfer. On the camera, manually selecting the white balance or the light source must be possible. Moreover, the camera should have optional software for presenting images on a computer monitor for checking details of images before and after they are captured. If a direct connection is not possible, a camera with a large memory card/stick capable of holding large, high-resolution files is recommended.
Given access to a height-adjustable tripod, lenses should be 50/55 mm. Lenses with a macro function can be useful for small objects but are not always necessary. Zoom lenses can be used but may have insufficient image sharpness. In addition to auto-focus, manual focus may be needed to capture sharp images of selected areas. Attaching polarized or UV filters to lenses for reducing gloss is an option; however, these filters tend to change the surface structure of the objects.
The cameras in modern smartphones can produce photos of high quality. Until now, a disadvantage with phone cameras has been the lack of available RAW format (the digital information collected that the computer interprets to generate an image for later image editing). All other formats are the processed data and thus contain less information. As with any camera, excellent focus and lack of blurring is a major goal.
Time-lapse cameras or cameras connected to a personal computer with this function can be useful to show changes in meat color over time. Stable, appropriate lighting is important. The frequency of photos and other settings can be adjusted according to needs. When conducting photography of meat, make sure to document technical information such as camera, lens, lighting, and more so that the photos can be accurately repeated later.
For best results with meat photography, use a camera that can capture images in a RAW file format. Digital cameras save photos in several file formats, including RAW, JPEG, or TIFF, with RAW being the format most preferred by many photographers. RAW images, also known as “digital negatives,” are almost unprocessed data coming directly from the camera sensor. These files preserve the most amount of information about an image and generally contain more colors and dynamic range than other formats. Unlike JPEG files, which can be opened easily, viewed, and printed by most image-viewing and editing programs, RAW is a proprietary format that is tied to the camera manufacturer and sensor; therefore, RAW files cannot be opened by some software packages.
With a new camera, it can take time for software companies to update their software. Because RAW files cannot be modified by third-party software, settings will have to be stored in a separate sidecar (XMP) file, which means more storage and tougher file management. Thumbnails can be used for simple recognition of RAW images. RAW formatting will capture up to 12 bits per color (red, blue, green, for 36 bits per location), whereas JPEG files can capture exactly 8 bits per color. Thus, RAW images will allow much greater post-camera processing capabilities to maximize the desired color regardless of whether the color is optimal or some stage of discoloration.
Other considerations
A tripod to support the camera and adjust the distance from the camera to the object is important for securing high-quality, clear images. A remote control for the camera is valuable to avoid vibrations and unclear images.
Meat should be photographed together with color and gray-tone patches (see Appendix E), preferably the first and last image of a series (Figure 20). Numerous companies make color and gray separation cards. In addition, X-Rite has a color checker system that might be useful. Color patch systems must be compatible with color adjustment software, such as Adobe Digital Negative Specification (DNG), and image processing software. Be aware that the lightness of the image must be as correct as possible at the time of exposure, because later major adjustment of lightness with image processing is difficult.
Many photo processing, rasterizing, and editing software packages are available. Usually, it is best to use whatever software is best suited to the camera. Recommended software for cameras can be found at the time the camera is purchased. With so much variation within camera brands as among camera types, the same is true for software. Adobe Photoshop, Elements, and Lightroom are popular photo management programs. Other free software is available on the internet, such as Corel PaintShop Pro and Fast Stone Image Viewer.
Troubleshooting the undesirable appearance of meat packages is greatly enhanced if good photographs that are in good focus are available. Often these photographs are taken in low light or in light that does not compliment the natural appearance of the meat. If possible, move the meat to a photographic-friendly area. Standardize the background (white wrapping paper, cardboard, metal trays or tables) and then shoot several views of the problem at hand. Try to take photos using available light in the room or on benches. If possible, adjust the white balance on the camera to complement the existing lighting. Shoot pictures with and without flash. Saving photos of the product both with and without a label (identification number, temperature, gas type, etc.) can be useful. If possible, also take a picture of the package or box label, which has a code that may be useful for solving the problem. Check the gas composition of the modified atmosphere packages in question, because it can greatly affect the color of the products. Look for leaks in package sealing, mark these clearly, and document possible defects with photos.
Meat photography often involves conditions ranging from hot temperatures to damp and dimly lighted areas to freezers—all of which can be harmful to film, digital cameras, and supplies. An insulated container with a good-sealing lid that can store all photo gear provides an excellent way to get gear into and out of coolers without rapid, drastic changes in humidity and without cold or warm temperatures that can lead to condensation in and on the surfaces of cameras, lens, filters, some media cards, etc. Cooling of the equipment is not as critical as avoiding sudden re-warming conditions. Use of the cooler is a great way to allow all gear to equilibrate gradually. Although not as good as a cooler, placing the sensitive gear in a large meat bag (double bagging is even better) that contains trapped air before tying off the bag is another way to transition photo equipment into and out of harsh environments. In addition, extra batteries and media cards should be carried in an interior pocket or pouch because camera batteries and cards may be less functional in cold environments.
Acknowledgements
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The authors are grateful to Jody Gallagher of the U.S. Meat Animal Research Center for her secretarial assistance. USDA is an equal opportunity provider and employer.
Literature Cited
Alderton, A. L., C. Faustman, D. C. Liebler, and D. W.Hill. 2003. Induction of redox instability of bovine myoglobin by adduction with 4-hydroxy-2-nonenal. Biochemistry-US. 42:4398–4405. doi: https://doi.org/10.1021/bi0271695.
AMSA. 2016. Research guidelines for cookery, sensory evaluation, and instrumental tenderness measurements of meat. 2 ed. Champaign, IL.
AOAC. 1990. Nitrites in cured meats. Method 973.31. In: K. Helrich, editor, Official methods of analysis. 15th ed. AOAC, Arlington, VA. p. 938.
Ashmore, C. R., W. Parker, and L. Doerr. 1972. Respiration of mitochondria isolated from dark cutting beef: Postmortem changes. J. Anim. Sci. 34:46–48. doi: https://doi.org/10.2527/JAS1972.34146X.
ASTM Committee E-18. 1968a. Basic principles of sensory evaluation. STP 433. Am. Soc. Testing Materials, Philadelphia, PA.
ASTM Committee E-18. 1968b. Manual on sensory testing methods. STP 434. Am. Soc. Testing Materials, Philadelphia, PA.
ASTM Committee E-18. 1978. Definition of terms relating to sensory evaluation of materials and products. E 253. Am. Soc. Testing Materials, Philadelphia, PA.
ASTM Committee E-18. 1979. ASTM manual on consumer sensory evaluation. STP 682. Am. Soc. Testing Materials, Philadelphia, PA.
ASTM Committee E-18. 1981. Guidelines for the selection and training of sensory panel members. STP 758. Am. Soc. Testing Materials, Philadelphia, PA.
Atkinson, J. L., and M. J. Follett. 1973. Biochemical studies on the discoloration of fresh meat. Int. J. Food Sci. Tech. 8:51–58. doi: https://doi.org/10.1111/j.1365-2621.1973.tb01688.x.
Ballard, C. M. 2004. Thermal denaturation of carboxymyoglobin and the effect of carbon monoxide, pH, and injection enhancement on display and cooked color of beef. M.S. thesis, Kansas State University, Manhattan, KS.
Barbut, S. 2001. Effect of illumination source on the appearance of fresh meat cuts. Meat Sci. 59:187–191.
Barton, P. A. 1967a. Measurement of colour stability of cooked cured meats to light and air. I. Development of method. J. Sci. Food Agr. 18:298–304. doi: https://doi.org/10.1002/jsfa.2740180709.
Barton, P. A. 1967b. Measurement of colour stability of cooked cured meats to light and air. II. Testing the procedure. J. Sci. Food Agr. 18:305–307. doi: https://doi.org/10.1002/jsfa.2740180710.
Bekhit, A. E. D., and C. Faustman. 2005. Metmyoglobin reducing activity. Meat Sci. 71:407–439. doi: https://doi.org/10.1016/j.meatsci.2005.04.032.
Bendall, J. R., and A. A. Taylor. 1972. Consumption of oxygen by the muscles of beef animals and related species. Consumption of oxygen by post-rigor muscles. J. Sci. Food Agr. 23:707–719. doi: https://doi.org/10.1002/jsfa.2740230606.
Bernofsky, C., J. B. Fox, and B. S. Schweigert. 1959. Biochemistry of myoglobin. VII. The effect of cooking on myoglobin in beef muscle. Adv. Food Res. 24:339. doi: https://doi.org/10.1111/j.1365-2621.1959.tb17281.x.
Bodwell, C. E., A. M. Pearson, and R. A. Fennell. 1965. Post-mortem changes in muscle III. Histochemical observations in beef and pork. J. Food Sci. 30:944–954. doi: https://doi.org/10.1111/j.1365-2621.1965.tb01869.x.
Böhner, N., and K. Rieblinger. 2016. Impact of different visible light spectra on oxygen absorption and surface discoloration of bologna sausage. Meat Sci. 121:207–209. doi: https://doi.org/10.1016/j.meatsci.2016.06.019.
Bowen, W. J. 1949. The absorption spectra and extinction coefficients of myoglobin. J. Biol. Chem. 179:235–245.
Buege, J. A., and S. D. Aust. 1978. Microsomal lipid peroxidation. Method. Enzymol. 52:302–304. doi: https://doi.org/10.1016/S0076-6879(78)52032-6.
Carpenter, C. E., and E. M. Clark. 1995. Evaluation of methods used in meat iron analysis and iron content of raw and cooked meats. J. Agr. Food Chem. 43:1824–1827.
Carter, P. 1971. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 40:450–458. doi: https://doi.org/10.1016/0003-2697(71)90405-2.
Cheah, K. S., and A. M. Cheah. 1971. Post-mortem changes in structure and function of ox muscle mitochondria. I. Electron microscopic and polarographic investigations. J. Bioenerg. Biomembr. 85–92. doi: https://doi.org/10.1007/BF01648923.
CIE (Commission Internationale de l’Eclairage). 1976. Recommendations on uniform color spaces—color difference equations, psychometric color terms. Supplement No. 2 to CIE Publication No. 15 (E-1.3.1.) 1978, 1971/(TC-1-3). Commission Internationale de l’Eclairage, Paris, France.
Cornforth, D. P. 2001. Spectrophotometric and reflectance measurements of pigments of cooked and cured meats [Unit F3.2]. In: S. J. Schwartz, editor, Current protocols in food analytical chemistry. John Wiley & Sons, Inc., New York, NY.
Cornforth, D., and K. Allen. 2009. Myoglobin oxidation reaction mechanism: Implications for fresh meat packaging. Paper presented at: Reciprocal Meats Conference, Rogers, AR.
Cornforth, D. P., and W. R. Egbert. 1985. Effect of rotenone and pH on the color of pre-rigor muscle. J. Food Sci. 50:34–35. doi: https://doi.org/10.1111/j.1365-2621.1985.tb13271.x.
Cornforth, D. P., J. K. Rabovitser, S. Ahuja, J. C. Wagner, R. Hanson, B. Cummings, and Y. Chudnovsky. 1998. Carbon monoxide, nitric oxide, and nitrogen dioxide levels in gas ovens related to surface pinking of cooked beef and turkey. J. Agr. Food Chem. 46:255–261. doi: https://doi.org/10.1021/jf970475i.
de Duve, C. 1948. A spectrophotometric method for the simultaneous determination of myoglobin and hemoglobin in extracts of human muscles. Acta Chem. Scand. 2:264. doi: https://doi.org/10.3891/acta.chem.scand.02-0264.
DeVore, D. P., and M. Solberg. 1975. A study of the rate-limiting factors in the respiratory oxygen consumption of intact post-rigor bovine muscle. J. Food Sci. 40:651–652.
Doane, T. A., and W. R. Horwáth. 2003. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 36:2713–2722. doi: https://doi.org/10.1081/AL-120024647.
Drabkin, D. L. 1950. The distribution of the chromoproteins, hemoglobin, myoglobin, and cytochrome c in the tissue of different species, and the relationship of the total content of each to body mass. J. Biol. Chem. 182:317–333.
Du, Z., and W. J. Bramlage. 1992. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plat tissue extracts. J. Agr. Food Chem. 40:1566–1570. doi: https://doi.org/10.1021/jf00021a018.
Erdman, A. M., and B. M. Watts. 1957. Spectrophotometric determination of color change in cured meat. J. Agr. Food Chem. 5:453–455. doi: https://doi.org/10.1021/jf60076a008.
Faustman, C., and R. G. Cassens. 1990. The biochemical basis for discoloration in fresh meat: A review. J. Muscle Foods 1:217–243. doi: https://doi.org/10.1111/j.1745-4573.1990.tb00366.x.
Faustman, C., and A. Phillips. 2001. Measurement of discoloration in fresh meat [Unit F3.3]. In: S. J. Schwartz, editor, Current protocols in food analytical chemistry. John Wiley & Sons, Inc., New York, NY. doi: https://doi.org/10.1002/0471142913.faf0303s00.
Ferreira, L. F., D. K. Townsend, B. J. Lutjemeier, and T. J. Barstow. 2005. Muscle capillary blood flow kinetics estimated from pulmonary oxygen uptake and near-infrared spectroscopy. J. Appl. Physiol. 98:1820–1828. doi: https://doi.org/10.1152/japplphysiol.00907.2004.
Georgala, D. L., and C. M. Davidson. 1970. Food Package. British patent I:199,998.
Ghorpade, V. M., and D. P. Cornforth. 1993. Spectra of pigments responsible for pink color in pork roasts cooked to 65 or 82°C. J. Food Sci. 58:51–52. doi: https://doi.org/10.1111/j.1365-2621.1993.tb03209.x.
Giddings, G. G. 1977. The basis of color in muscle foods. CRC Cr. Rev. Food Sci. 9:81–114. doi: https://doi.org/10.1080/10408397709527231.
Giddings, G. G., and H. O. Hultin. 1974. Reduction of ferrimyoglobin in meat. CRC Cr. Rev. Food Sci. 2:143–173.
Greene, B. E., and T. H. Cumuze. 1981. Relationship between TBA numbers and inexperienced panelists’ assessment of oxidized flavor in cooked beef. J. Food Sci. 47:52–54, 58.
Greene, B. E., I. Hsin, and M. W. Zipser. 1971. Retardation of oxidative color changes in raw ground beef. J. Food Sci. 36:940–942. doi: https://doi.org/10.1111/j.1365-2621.1982.tb11025.x.
Grunwald, E. W., and M. P. Richards. 2006. Studies with myoglobin variants indicate that released hemin is the primary promoter of lipid oxidation in washed fish muscle. J. Agr. Food Chem. 54:4452–4460. doi: https://doi.org/10.1021/jf0603228.
Hagler, L., R. I. Coppes Jr., and R. H. Herman. 1979. Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. J. Biol. Chem. 254:6505–6514.
Hague, M. A., K. E. Warren, M. C. Hunt, D. H. Kropf, C. L. Kastner, S. L. Stroda, and D. E. Johnson. 1994. Endpoint temperature, internal cooked color, and expressible juice color relationships in ground beef patties. J. Food Sci. 59:465–470. doi: https://doi.org/10.1111/j.1365-2621.1994.tb05539.x.
Heaton, K. M., D. P. Cornforth, I. V. Moiseev, W. R. Egbert, and C. E. Carpenter. 2000. Minimum sodium nitrite levels for pinking of various cooked meats as related to use of direct or indirect-dried soy isolates in poultry rolls. Meat Sci. 55:321–329. doi: https://doi.org/10.1016/s0309-1740(99)00160-6.
Hodgen, J. 2016. Comparison of nix color sensor and nix color sensor pro to standard meat science research colorimeters. Meat Sci. 112:159. doi: https://doi.org/10.1016/j.meatsci.2015.08.129.
Holman, B. W. B., D. Collins, A. K. Kilgannon, and D. L. Hopkins. 2018. The effect of technical replicate (repeats) on Nix Pro Color Sensor measurement precision for meat: A case-study on aged beef colour stability. Meat Sci. 135:42–45. doi: https://doi.org/10.1016/j.meatsci.2017.09.001.
Hood, D. E. 1980. Factors affecting the rate of metmyoglobin accumulation in pre-packaged beef. Meat Sci. 4:247–265. doi: https://doi.org/10.1016/0309-1740(80)90026-1.
Hornsey, H. C. 1956. Color of cooked cured pork. I. Estimation of the nitric oxide-haem pigments. J. Sci. Food Agr. 23:534–540. doi: https://doi.org/10.1002/jsfa.2740070804.
Howe, J. L., E. A. Gullett, and W. R. Usborne. 1982. Development of pink color in cooked pork. Can. Inst. F. Sci. Tec. J. 15:19. doi: https://doi.org/10.1016/S0315-5463(82)72308-9.
Hunt, M. C., and H. B. Hedrick. 1977. Chemical, physical, and sensory characteristics of bovine muscle from four quality groups. J. Food Sci. 42:716–720. doi: https://doi.org/10.1111/j.1365-2621.1977.tb12586.x.
Hunt, M. C., O. Sørheim, and E. Slinde. 1999. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 64:847–851. doi: https://doi.org/10.1111/J.1365-2621.1999.TB15925.X.
IFT Sensory Evaluation Division. 1995. Policies for the preparation and review of papers reporting sensory evaluation data. J. Food Sci. 60:210. doi: https://doi.org/10.1111/j.1365-2621.1995.tb05639.x.
Igene, J. O., K. Yamauchi, A. M. Pearson, J. I. Gray, and S. D. Aust. 1985. Mechanisms by which nitrite inhibits the development of warmed-over flavour (WOF) in cured meat. Food Chem. 18:1–18. doi: https://doi.org/10.1016/0308-8146(85)90099-8.
Jayasingh, P. 2004. Prevention of pigment deterioration and lipid oxidation in ground beef and pork. Ph.D. diss., Utah State University, Logan, UT.
Joseph, P., S. P. Suman, S. Li, C. M. Beach, and J. R. Claus. 2010a. Mass spectrometric characterization and thermo-stability of turkey myoglobin. Lebensm. Wiss. Technol. 43:273–278. doi: https://doi.org/10.1016/j.lwt.2009.08.019.
Karlsson, A., and K. Lundstrom. 1991. Meat pigment determination by a simple and non-toxic alkaline haematin method (an alternative to the Hornsey and the cyanometmyoglobin methods). Meat Sci. 29:17–24. doi: https://doi.org/10.1016/0309-1740(91)90019-M.
Killday, B. K., M. S. Tempesta, M. E. Bailey, and C. J. Metral. 1988. Structural characterization of nitrosylhemochromogen of cooked cured meat: Implications in the meat curing reaction. J. Agr. Food Chem. 36:909–914. doi: https://doi.org/10.1021/jf00083a006.
Killinger, K. M., M. C. Hunt, and R. E. Campbell. 2000. Factors affecting premature browning during cooking of ground beef purchased at retail. J. Food Sci. 65:585–587. doi: https://doi.org/10.1111/j.1365-2621.2000.tb16053.x.
Kim, Y. H., M. C. Hunt, R. A. Mancini, M. Seyfert, T. M. Loughin, D. H. Kropf, and J. S. Smith. 2006. Mechanism for lactate-color stabilization in injection-enhanced beef. J. Agr. Food Chem. 54:7856–7862. doi: https://doi.org/10.1021/jf061225h.
King, D. A., S. D. Shackelford, A. B. Rodriguez, and T. L. Wheeler. 2011. Effect of time of measurement on the relationship between metmyoglobin reducing activity and oxygen consumption to instrumental measures of beef longissimus color stability. Meat Sci. 87:26–32. doi: https://doi.org/10.1016/j.meatsci.2010.08.013.
Kinnear, P. R., and A. Sahraie. 2002. New Farnsworth-Munsell 100-Hue test norms of normal observers for each year of age 5–22 and for age decades 30–70. Brit. J. Ophthalmol. 8:1408–1411. doi: https://doi.org/10.1136/bjo.86.12.1408.
Knock, R. C., M. Seyfert, M. C. Hunt, M. E. Dikeman, R. A. Mancini, J. A. Unruh, J. J. Higgins, and R. A. Monderen. 2006. Effects of potassium lactate, sodium chloride, sodium tripolyphosphate, and sodium acetate on colour, colour stability, and oxidative properties of injection-enhanced beef rib steaks. Meat Sci. 74:312–318. doi: https://doi.org/10.1016/j.meatsci.2006.03.029.
Kropf, D. H. 2004. Packaging: Vacuum. In: W. K. Jensen, C. Devine, and M. Dikeman, editors, Encyclopedia of meat sciences. Elsevier Acad. Press, Oxford. p. 956.
Krzywicki, K. 1979. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. doi: https://doi.org/10.1016/0309-1740(79)90019-6.
Krzywicki, K. 1982. The determination of haem pigments in meat. Meat Sci. 7:29–36. doi: https://doi.org/10.1016/0309-1740(82)90095-X.
Lanari, M. C., and R. G. Cassens. 1991. Mitochondrial activity and beef muscle color stability. J. Food Sci. 56:1476–1479. doi: https://doi.org/10.1111/j.1365-2621.1991.tb08619.x.
Landrock, A. H., and G. A. Wallace. 1955. Discoloration of fresh red meat and its relationship to film oxygen permeability. Food Technol.-Chicago. 9:194.
Larsen, H., F. Westad, O. Sørheim, and L. H. Nilsen. 2006. Determination of critical oxygen level in packages for cooked sliced ham to prevent color fading during illuminated retail display. J. Food Sci. 71:S407–S413. doi: https://doi.org/10.1111/j.1750-3841.2006.00048.x.
Lavelle, C., M. C. Hunt, and D. H. Kropf. 1995. Display life and internal cooked color of ground beef from vitamin E-supplemented steers. J. Food Sci. 60:1–4. doi: https://doi.org/10.1111/j.1365-2621.1995.tb04549.x.
Lawrence, T. E., M. C. Hunt, and D. H. Kropf. 2002b. Surface roughening of pre-cooked, cured beef round muscles reduces iridescence. J. Muscle Foods. 13:69–73. doi: https://doi.org/10.1111/j.1745-4573.2002.tb00321.x.
Lawrence, T. E., M. C. Hunt, E. Obuz, D. Kropf, and H. Wang. 2002a. Muscle iridescence: Potential causes—Possible solutions. Pages 25–30 in Proceedings of the 48th International Congress of Meat Science and Technology, Rome, Italy, August.
Ledward, D. A. 1971. On the nature of cooked meat hemoprotein. J. Food Sci. 36:883–888. doi: https://doi.org/10.1111/j.1365-2621.1971.tb15552.x.
Lyon, B. G., B. E. Greene, and C. E. Davis. 1986. Color, doneness and soluble protein characteristics of dry roasted beef semitendinosus. J. Food Sci. 51:24–27. doi: https://doi.org/10.1111/j.1365-2621.1986.tb10827.x.
MacDougall, D. B. 1982. Changes in the color and opacity of meat. Food Chem. 9:75–88. doi: https://doi.org/10.1016/0308-8146(82)90070-X.
Madhavi, D. L., and C. E. Carpenter. 1993. Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J. Food Sci. 58:939–942. doi: https://doi.org/10.1111/j.1365-2621.1993.tb06083.x.
Mancini, R. A., and M. C. Hunt. 2005. Current research in meat color. Meat Sci. 71:100–121. doi: https://doi.org/10.1016/j.meatsci.2005.03.003.
Mancini, R. A., M. Seyfert, and M. C. Hunt. 2008. Effects of data expression, sample location, and oxygen partial pressure on initial nitric oxide metmyoglobin formation and metmyoglobin-reducing-activity measurement in beef muscle. Meat Sci. 79:244–251. doi: https://doi.org/10.1016/j.meatsci.2007.09.008.
Mancini, R. A., S. P. Suman, M. K. R. Konda, and R. Ramanathan. 2009. Effect of carbon monoxide packaging and lactate enhancement on the color stability of beef steaks stored at 1°C for 9 days. Meat Sci. 81:71–76. doi: https://doi.org/10.1016/j.meatsci.2008.06.021.
Mancini, R. A., S. P. Suman, M. K. R. Konda, R. Ramanathan, P. Joseph, and C. M. Beach. 2010. Mass spectrometric investigations on lactate adduction to equine myoglobin. Meat Sci. 85:363–367. doi: https://doi.org/10.1016/j.meatsci.2010.02.006.
Marcuse, R., and L. Johansson. 1973. Studies on the TBA test for rancidity grading: II. TBA reactivity of different aldehyde classes. J. Am. Oil Chem. Soc. 50:387–391. doi: https://doi.org/10.1007/BF02641814.
Marksberry, C. L. 1992. Effect on fat level, pH, carcass maturity, and compaction on cooked internal color of ground beef patties at five endpoint temperatures. M.S. thesis, Kansas State University, Manhattan, KS.
McKenna, D. R., P. D. Mies, B. E. Baird, K. D. Pfeiffer, J. W. Ellebracht, and J. W. Savell. 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682. doi: https://doi.org/10.1016/j.meatsci.2005.02.016.
McLellan, M. R., L. R. Lind, and R. W. Kime. 1995. Hue angle determinations and statistical analysis for multi-quadrant Hunter L,a,b, data. J. Food Quality 18:235–240. doi: https://doi.org/10.1111/j.1745-4557.1995.tb00377.x.
Meilgaard, M. C., G. V. Civille, and B. T. Carr. 1991. Sensory evaluation techniques. CRC Press Inc., Boca Raton, FL.
Miller, R. K. 1994. Sensory methods to evaluate muscle foods. In: D. M. Kinsman and A. W. Kotula, editors, Muscle foods. Chapman and Hall Publ., New York, NY.
Miranda, K. M., M. G. Espey, and D. A. Wink. 2001. A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71. doi: https://doi.org/10.1006/niox.2000.0319.
Møeller, J. K. S., M. Jakobsen, C. J. Weber, T. Martinussen, L. H. Skibstead, and G. Bertelsen. 2003. Optimisation of colour stability of cured ham during packaging and retail display by a multifactorial design. Meat Sci. 63:169–175. doi: https://doi.org/10.1016/S0309-1740(02)00066-9.
Møeller, J. K. S., L. Nannerup, and L. H. Skibstead. 2004. Effect of carbon dioxide on autoxidation and photooxidation of nitrosylmyoglobin. Meat Sci. 69:71–78. doi: https://doi.org/10.1016/j.meatsci.2004.06.007.
Mohan, A., M. C. Hunt, T. J. Barstow, T. A. Houser, and D. M. Hueber. 2010a. Near-infrared oximetry of three post-rigor skeletal muscles for following myoglobin redox forms. Food Chem. 123:456–464. doi: https://doi.org/10.1016/j.foodchem.2010.04.068.
Mohan, A., M. C. Hunt, T. J. Barstow, T. A. Houser, and S. Muthukrishnan. 2010b. Effects of lactate, malate, and pyruvate on myoglobin redox stability of three bovine muscles. Meat Sci. 86:304–310. doi: https://doi.org/10.1016/j.meatsci.2010.04.030.
Mohan, A., M. C. Hunt, S. Muthukrishnan, T. A. Houser, and T. E. Barstow. 2010c. Myoglobin redox form stabilization by compartmentalized lactate and malate dehydrogenases. J. Agr. Food Chem. 58:7021–7029. doi: https://doi.org/10.1021/jf100714g.
Moiseev, I. V., and D. P. Cornforth. 1999. Treatments for prevention of persistent pinking in dark-cutting beef patties. J. Food Sci. 64:738–743. doi: https://doi.org/10.1111/j.1365-2621.1999.tb15122.x.
Morita, H., J. Niu, R. Sakata, and Y. Nagata. 1996. Red pigment of Parma ham and bacterial influence on its formation. J. Food Sci. 61:1021–1023. doi: https://doi.org/10.1111/j.1365-2621.1996.tb10924.x.
Nam, K. C., and D. U. Ahn. 2002. Carbon monoxide-heme pigment is responsible for the pink color in irradiated raw turkey breast meat. Meat Sci. 60:25–33. doi: https://doi.org/10.1016/s0309-1740(01)00101-2.
O’Keeffe, M., and D. E. Hood. 1982. Biochemical factors influencing metmyoglobin formation on beef from muscles of differing colour stability. Meat Sci. 7:209–228. doi: https://doi.org/10.1016/0309-1740(82)90087-0.
O’Sullivan, M. G., and J. P. Kerry. 2010. Meat packaging [Ch. 13]. In: F. Toldra, editor, Handbook of meat processing. Wiley-Blackwell, London. p. 249.
Oellingrath, I. M., A. Iversen, and G. Skrede. 1990. Quantitative determination of myoglobin and haemoglobin in beef by high-performance liquid chromatography. Meat Sci. 28:313–320. doi: https://doi.org/10.1016/0309-1740(90)90045-8.
Pearson, A. M., and F. W. Tauber. 1984. Analytical methods. In: A. M. Pearson and F. W. Tauber, editors, Processed meats. 2nd ed. AVI Publ., Westport, CT. pp. 360–361.
Raines, C. R., and M. C. Hunt. 2010. Effects of headspace volume and molecular availability of carbon monoxide on carboxymyoglobin development of modified atmosphere packaged beef steaks. J. Food Sci. 75:C62–C65. doi: https://doi.org/10.1111/j.1750-3841.2009.01418.x.
Ramanathan, R., R. A. Mancini, and M. K. Konda. 2009. Effects of lactate on beef heart mitochondrial oxygen consumption and muscle darkening. J. Agr. Food Chem. 57:1550–1555. doi: https://doi.org/10.1021/jf802933p.
Renerre, M., and R. Labas. 1987. Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Sci. 19:151–165. doi: https://doi.org/10.1016/0309-1740(87)90020-9.
Sammel, L. M., M. C. Hunt, D. H. Kropf, K. A. Hachmeister, C. L. Kastner, and D. E. Johnson. 2002a. Comparison of assays for metmyoglobin reducing ability in beef inside and outside semimembranosus. J. Food Sci. 67:978–984. doi: https://doi.org/10.1111/j.1365-2621.2002.tb09439.x.
Sammel, L. M., M. C. Hunt, D. H. Kropf, K. A. Hachmeister, C. L. Kastner, and D. E. Johnson. 2002b. Influence of chemical characteristics of beef inside and outside semimembranosus on color traits. J. Food Sci. 67:1323–1330. doi: https://doi.org/10.1111/j.1365-2621.2002.tb10282.x.
Schricker, B. R., D. D. Miller, and J. R. Stouffer. 1982. Measurement and content of nonheme and total iron in muscle. J. Food Sci. 47:740–743. doi: https://doi.org/10.1111/j.1365-2621.1982.tb12704.x.
Schwarz, S. J., J. R. Claus, H. Wang, N. B. Marriott, and P. P. Graham. 1998. Quantification of nicotinamide hemochrome in cooked, uncured turkey by reflectance spectrophotometry. J. Muscle Foods. 9:101–110.
Sen, N. P., and B. Donaldson. 1978. Improved colorimetric method for determining nitrate and nitrite levels in foods. J. Assoc. Off. Ana. Chem. 61:1389–1394.
Sen, N. P., and Y. C. Lee. 1979. Determination of nitrate and nitrite in whey powder. J. Agr. Food Chem. 27:1277–1279. doi: https://doi.org/10.1021/jf60226a043.
Setser, C. S. 1984. Color: Reflections and transmissions. J. Food Quality 6:183–197.
Shahidi, F., L. J. Rubin, L. L. Diosady, and D. F. Wood. 1985. Effect of sulfanilamide on the TBA values of cured meats. J. Food Sci. 50:274–275.
Shikama, K., and Y. Sugawara. 1978. Autoxidation of native oxymyoglobin. Kinetic analysis of the pH profiles. Eur. J. Biochem. 91:407–413. doi: https://doi.org/10.1111/j.1432-1033.1978.tb12693.x.
Siegel, D. G. 2007. Packaging for processed meats. In: W. N. Osburn, G. Sebranek, and C. M. Schultz Kaster, editors, Meat processing technology series. Am. Meat Sci. Assoc., Champaign, IL. pp. 31–34.
Siegel, D. 2009. Vacuum packaging fresh meat with nitrite containing film. Paper presented at: Proceedings of the Reciprocal Meats Conference, Rogers, AR, June 22.
Sørheim, O., Ø. Langsrud, D. P. Cornforth, T. C. Johannessen, E. Slinde, P. Berg, and T. Nesbakken. 2006. Carbon monoxide as a colorant in cooked or fermented sausages. J. Food Sci. 71:C549–C555. doi: https://doi.org/10.1111/j.1750-3841.2006.00183.x.
Strange, E. D., R. C. Benedict, R. E. Gugger, V. G. Metzger, and C. E. Swift. 1974. Simplified methodology for measuring meat color. J. Food Sci. 39:988–992.
Suman, S. P., P. Joseph, S. Li, C. M. Beach, L. Steinke, and M. Fontaine. 2010. Amino acid sequence of myoglobin from emu (Dromaius novaehollandiae) skeletal muscle. Meat Sci. 86:623–628. doi: https://doi.org/10.1016/j.meatsci.2010.04.041.
Suman, S. P., R. A. Mancini, and C. Faustman. 2006. Lipid-oxidation-induced carboxymyoglobin oxidation. J. Agr. Food Chem. 54:9248–9253. doi: https://doi.org/10.1021/jf061959u.
Suman, S. P., R. A. Mancini, R. Ramanathan, and M. R. Konda. 2009. Effect of lactate-enhancement, modified atmosphere packaging, and muscle source on the internal cooked color of beef steaks. Meat Sci. 81:664–670. doi: https://doi.org/10.1016/j.meatsci.2010.02.001.
Tang, J., C. Faustman, and T. A. Hoagland. 2004. Krzywicki revisited: Equations for spectrophotometric determination of myoglobin redox forms in aqueous meat extracts. J. Food Sci. 69:C717–C720. doi: https://doi.org/10.1111/j.1365-2621.2004.tb09922.x.
Tang, J., C. Faustman, T. A. Hoagland, R. A. Mancini, M. Seyfert, and M. C. Hunt. 2005a. Interactions between mitochondrial lipid oxidation and oxymyoglobin oxidation and the effects of vitamin E. J. Agr. Food Chem. 53:6073–6079. doi: https://doi.org/10.1021/jf0501037.
Tang, J., C. Faustman, T. A. Hoagland, R. A. Mancini, M. Seyfert, and M. C. Hunt. 2005b. Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J. Agr. Food Chem. 53:1223–1230. doi: https://doi.org/10.1021/jf048646o.
Tang, J., C. Faustman, R. A. Mancini, M. Seyfert, and M. C. Hunt. 2005c. Mitochondrial reduction of metmyoglobin: Dependence on the electron transport chain. J. Agr. Food Chem. 53:5449–5455. doi: https://doi.org/10.1021/jf050092h.
Tapp, W. H., III, Y. W. S. Yancey, and J. K. Apple. 2011. How is the instrumental color of meat measured? Meat Sci. 89:1–5. doi: https://doi.org/10.1016/j.meatsci.2010.11.021.
Tappel, A. L. 1957. Reflectance spectral studies of the hematin pigments of cooked beef. Adv. Food Res. 22:404.
Tarladgis, B. G., B. M. Watts, and M. T. Younathan. 1960. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 37:44–48. doi: https://doi.org/10.1007/BF02630824.
Trout, G. R. 1989. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J. Food Sci. 54:536. doi: https://doi.org/10.1111/j.1365-2621.1989.tb04644.x.
Trout, G. R., and D. A. Gutzke. 1996. A simple, rapid preparative method for isolating and purifying oxymyoglobin. Meat Sci. 43:1–13. doi: https://doi.org/10.1016/0309-1740(95)00068-2.
Urbin, M. C., and G. D. Wilson. 1961. The post-mortem oxygen requirements of bovine tissue. J. Food Sci. 26:313–317.
Vasavada, M. N., and D. P. Cornforth. 2006. Evaluation of antioxidant effects of raisin paste in cooked ground beef, pork, and chicken. J. Food Sci. 71:C247–C254. doi: https://doi.org/10.1111/j.1750-3841.2006.00026.x.
Wakamatsu, J., T. Nishimura, and A. Hattori. 2004. A Zn-porphyrin complex contributes to bright red color in Parma ham. Meat Sci. 67:95–100. doi: https://doi.org/10.1016/j.meatsci.2003.09.012.
Warriss, P. D. 1979. The extraction of haem pigments from fresh meat. Int. J. Food Sci. Tech. 14:75–80. doi: https://doi.org/10.1111/j.1365-2621.1979.tb00849.x.
Watanabe, A., Y. Ueda, M. Higuchi, and N. Shiba. 2008. Analysis of volatile compounds in beef fat by dynamic-headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. J. Food Sci. 73:C420–C425. doi: https://doi.org/10.1111/j.1750-3841.2008.00764.x.
Watts, B. M., J. Kendrick, M. W. Zipser, B. Hutchins, and B. Saleh. 1966. Enzymatic reducing pathways in meat. J. Food Sci. 32:855–862. doi: https://doi.org/10.1111/j.1365-2621.1966.tb03261.x.
Wiegand, C., and G. Waloszek. 2003. Color glossary A-C. http://www.sapdesignguild.org/resources/glossary color/index1.html#norm_cs. (Accessed 30 January 2011).
Witte, V. C., G. F. Krause, and M. E. Bailey. 1970. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 35:582–585. doi: https://doi.org/10.1111/j.1365-2621.1970.tb04815.x.
Yancey, J. W. S., and D. H. Kropf. 2008. Instrumental reflectance values of fresh pork are dependent on aperture size. Meat Sci. 79:734–739. doi: https://doi.org/10.1016/j.meatsci.2007.11.006.
Yin, M. C., and C. Faustman. 1993. Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid: A liposome model. J. Agr. Food Chem. 41:853–857. doi: https://doi.org/10.1021/jf00030a002.
Zipser, M. W., and B. M. Watts. 1962. A modified 2-thiobarbituric acid (TBA) method for the determination of malonaldehyde in cured meats. Food Technol.-Chicago 16:102–105.
Appendix A: Visual Scoring Scales for Meat
Visual-color scoring scales can be used for consumer-preference panels or for trained descriptive panels. Appropriate scales should be selected based on the panel objective. Hedonic scales are commonly used for consumer-preference panels to evaluate how much a consumer prefers the color and appearance of products on display. Trained descriptive-color panels often use more complex scales and characterize meat color, evaluate color throughout shelf life, and/or assess the amount of discoloration.
Several examples of scales that can be used for various products and packaging environments are provided. However, most scales, including those listed below and those selected from the literature, likely will need the descriptive terms modified to meet individual study objectives. Scoring scales for all visual-color panels must be selected and adapted to fit the uniqueness of the product and the objective being evaluated. Consequently, investigators must conduct preliminary experiments to see whether selected scales adequately characterize the specific changes observed in the experiment. During these sessions with panelists, scales must be anchored to actual, preliminary samples or pictorial references so all panelists use the scale similarly. Researchers must clearly and accurately communicate the scales developed and utilized when reporting data and interpreting results.
A.
These are scales used by consumer-preference panelists to characterize opinions, preferences, desirability, willingness to purchase, etc. Often, open-ended questions to accompany hedonic scales can help explain what consumers do or do not like about samples from a specific treatment. When using these types of panels and scales, the term “neutrality” must be included, so panelists are not forced to indicate a preference if they do not feel strongly one way or another.
| Color of Meat in This Package | Overall Meat Color Is |
|---|---|
| 7 = Like very much | 9 = Extremely desirable or acceptable color |
| 6 = Like moderately | 8 = Very desirable or acceptable color |
| 5 = Like slightly | 7 = Moderately desirable or acceptable color |
| 4 = Neutral | 6 = Slightly desirable or acceptable color |
| 3 = Dislike slightly | 5 = Neither acceptable nor unacceptable color |
| 2 = Dislike moderately | 4 = Slightly undesirable or unacceptable color |
| 1 = Dislike very much | 3 = Moderately undesirable or unacceptable color |
| 2 = Very undesirable or unacceptable color | |
| 1 = Extremely undesirable or unacceptable color |
| Based on Meat Color |
|---|
| 7 = Very definitely would purchase |
| 6 = Definitely would purchase |
| 5 = Probably would purchase |
| 4 = May or may not purchase |
| 3 = Probably would not purchase |
| 2 = Definitely would not purchase |
| 1 = Very definitely would not purchase |
| This Meat Has Desirable Color |
|---|
| 7 = Very strongly agree |
| 6 = Strongly agree |
| 5 = Slightly agree |
| 4 = No opinion |
| 3 = Slightly disagree |
| 2 = Strongly disagree |
| 1 = Very strongly disagree |
B.
These are scales used by descriptive-visual panelists to characterize meat color at the beginning of the experiment (such as Pale Soft Exudative, Dark Firm Dry, etc.).
Oxygenated or Carbon Monoxide Modified Atmosphere Packages
| Beef or Lamb | Pork or Poultry |
|---|---|
| 1 = Pale red | 1 = Pale grayish-pink |
| 2 = Slightly pale red | 2 = Slightly pale grayish-pink |
| 3 = Moderately light red | 3 = Moderately light grayish-pink |
| 4 = Bright red | 4 = Bright grayish-pink |
| 5 = Slightly dark red | 5 = Slightly dark grayish-pink |
| 6 = Moderately dark red | 6 = Moderately dark grayish-pink |
| 7 = Dark red | 7 = Dark grayish-pink |
| 8 = Very dark red | 8 = Very dark grayish-pink |
Note: Panelist can record scores to nearest 0.5 point.
Low-Oxygen Modified Atmosphere Packages
| Beef or Lamb | Pork or Poultry |
|---|---|
| 1 = Pale purple-red | 1 = Pale purplish-pink |
| 2 = Slightly pale purple-red | 2 = Slightly pale purplish-pink |
| 3 = Moderately light purple-red | 3 = Moderately light purplish-pink |
| 4 = Purple-red | 4 = Purplish-pink |
| 5 = Slightly dark purple-red | 5 = Slightly dark purplish-pink |
| 6 = Moderately dark purple-red | 6 = Moderately dark purplish-pink |
| 7 = Dark purple-red | 7 = Dark purplish-pink |
| 8 = Extremely dark purple-red | 8 = Extremely dark purplish-pink |
Note: Panelist can record scores to nearest 0.5 point.
C.
These are scales used to evaluate how meat color changes throughout shelf life.
Beef, Lamb, Pork, or Poultry in Oxygenated (≈20.6% O2) Packages
| Beef | Lamb | Pork or Poultry |
|---|---|---|
| 1 = Extremely bright cherry-red | 1 = Extremely bright brick-red | 1 = Very bright reddish pink |
| 2 = Bright cherry-red | 2 = Bright brick-red | 2 = Bright reddish pink |
| 3 = Moderately bright cherry-red | 3 = Moderately bright brick-red | 3 = Dull reddish pink |
| 4 = Slightly bright cherry-red | 4 = Slightly bright brick-red | 4 = Slightly grayish pink |
| 5 = Slightly dark cherry-red | 5 = Slightly dark brick-red | 5 = Grayish pink |
| 6 = Moderately dark reddish-tan | 6 = Moderately dark reddish-tan | 6 = Slightly tannish gray |
| 7 = Dark tan to brown | 7 = Dark tan to brown | 7 = Moderately tannish gray |
| 8 = Extremely dark brown | 8 = Extremely dark brown | 8 = Tan to brown |
Note: Panelist can record scores to nearest 0.5 point.
Beef, Lamb, Pork, or Poultry in High-Oxygen MAP or Carbon Monoxide MAP
| Beef or Lamb | Pork or Poultry |
|---|---|
| 1 = Very bright red | 1 = Very bright reddish pink |
| 2 = Bright red | 2 = Bright reddish pink |
| 3 = Dull red | 3 = Dull reddish pink |
| 4 = Slightly dark red | 4 = Slightly grayish pink |
| 5 = Moderately dark red | 5 = Moderately grayish pink |
| 6 = Dark red to dark reddish tan | 6 = Dark tannish gray |
| 7 = Tannish red | 7 = Tannish gray |
| 8 = Tan to brown | 8 = Tan to brown |
Note: Panelist can record scores to nearest 0.5 point.
MAP = modified atmosphere packaging.
Low-Oxygen Packages
| Beef | Lamb |
|---|---|
| 1 = Extremely bright purple-red | 1 = Extremely bright purplish-pink |
| 2 = Bright purple-red | 2 = Bright purplish-pink |
| 3 = Moderately bright purple-red | 3 = Moderately bright purplish-pink |
| 4 = Slightly purple-red | 4 = Slightly purplish-pink |
| 5 = Slightly dark purple | 5 = Slightly dark purplish-pink |
| 6 = Moderately dark purple | 6 = Moderately dark purplish-pink |
| 7 = Dark purple | 7 = Dark purplish-pink |
| 8 = Extremely dark purple | 8 = Extremely purplish-pink |
Note: Panelist can record scores to nearest 0.5 point.
Multiple-use scale for determining:
- a)
The conversion of oxymyoglobn (OMb) to metmyoglobin (MMb) to deoxymyoglobin (DMb) post vacuum packaging,
- b)
Meat appearance during vacuum storage, or
- c)
The blooming ability of meat after removal from the vacuum package.
Applicable for most species and for cuts with normal red to pale muscle portions.
1 = Bright red or bright pinkish red (color immediately after packaging or the degree of bloom)
2 = Dull red or dull pinkish red
3 = Slightly red to tannish red or pink
4 = Moderately tannish red or pink
5 = Tan to brown
6 = Slightly tannish purple
7 = Moderately purple
8 = Purple (typical vacuum package color)
Note: Panelist can score to nearest 0.5 point.
| Browning in Vacuum Packages | Percentage of MMb in Vacuum Packages |
|---|---|
| Applicable to beef, lamb, and pork | Applicable for most species |
| 1 = None, no tan or brown | 1 = 0% MMb |
| 2 = Slight amount of tan or brown | 2 = 1% to 20% MMb |
| 3 = Small amount of tan or brown | 3 = 21% to 40% MMb |
| 4 = Moderate amount of tan or brown | 4 = 41% to 60% MMb |
| 5 = Nearly all tan or brown | 5 = 61% to 99% MMb |
| Note: Panelist can score to nearest 0.5 point | 6 = 100% MMb |
The worst-point color is a single or combined area of at least 2 cm2 (or some other predetermined area). Score using the same scale used to evaluate “average” color. For example, if using the scale below and if the worst-point colored area was “slightly dark cherry red,” the worst-point color score would be a 5.0. The overall average score for the cut could still be a 2.5 excluding the worst-point colored area.
1 = Extremely bright cherry-red or bright red
2 = Bright cherry-red or bright red
3 = Moderately bright cherry-red or bright red
4 = Slightly bright cherry-red or bright red
5 = Slightly dark cherry-red or bright red
6 = Moderately dark red
7 = Dark red
8 = Extremely dark red
| Amount of Browning | Discoloration |
|---|---|
| 1 = No evidence of browning | 1 = None |
| 2 = Dull | 2 = Slight |
| 3 = Grayish | 3 = Small |
| 4 = Brownish-gray | 4 = Moderate |
| 5 = Brown | 5 = Extreme |
| 6 = Dark brown | Note: Panelist can record scores to nearest 0.5 point. |
| Note: Panelist can record scores to nearest 0.5 point. |
| Surface Discoloration (% MMb Formation) | Surface Discoloration (% MMb Formation) |
|---|---|
| 1 = No discoloration, 0% | 1 = No observable MMb, 0% |
| 2 = Slight discoloration, 1% to 20% | 2 = Slight amount of MMb, 1% to 15% |
| 3 = Small discoloration, 21% to 40% | 3 = Small amount of MMb, 16% to 30% |
| 4 = Modest discoloration, 41% to 60% | 4 = Moderate amount of MMb, 31% to 45% |
| 5 = Moderate discoloration, 61% to 80% | 5 = Extensive MMb, > 45% |
| 6 = Extensive discoloration, 81% to 100% | Note: Use this scale to determine how consumers often detect and discriminate against MMb. |
D.
Initial Color of Ground Meat
| Beef or Lamb | Pork or Poultry |
|---|---|
| 1 = Very light red | 1 = Very light grayish pink |
| 2 = Moderately light red | 2 = Moderately light grayish pink |
| 3 = Light red | 3 = Light grayish pink |
| 4 = Slightly bright red | 4 = Slightly bright grayish pink |
| 5 = Bright red | 5 = Bright grayish pink |
| 6 = Slightly dark red | 6 = Slightly dark grayish pink |
| 7 = Moderately dark red | 7 = Moderately dark grayish pink |
| 8 = Dark red | 8 = Dark grayish pink |
Note: Panelist can record scores to nearest 0.5 point.
Ground Product Display Discoloration
| Beef or Lamb | Pork or Poultry |
|---|---|
| 1 = Very bright red | 1 = Very bright reddish-pink |
| 2 = Bright red | 2 = Bright reddish-pink |
| 3 = Dull red | 3 = Dull reddish-pink |
| 4 = Slightly dark red | 4 = Slightly dark reddish-pink |
| 5 = Moderately dark red | 5 = Moderately reddish-pink |
| 6 = Dark red to tannish-red | 6 = Dark tannish-gray |
| 7 = Dark reddish-tan | 7 = Dark tannish-gray |
| 8 = Tan to brown | 8 = Tan to brown |
Note: Panelist can record scores to nearest 0.5 point.
E.
These are scales used by descriptive panelists to evaluate heating effects on meat color.
1 = Very red
2 = Slightly red
3 = Pink
4 = Slightly pink
5 = Pinkish-gray
6 = Grayish tan/brown
7 = Tan/brown
Note: Panelist can record scores to nearest 0.5 point.
1 = Very rare
2 = Rare
3 = Medium rare
4 = Medium
5 = Well done
6 = Very well done
Note: Panelist can record scores to nearest 0.5 point.
−3 = Moderately darker
−2 = Slightly darker
−1 = Very slightly darker
0 = Not different from control
1 = Very slightly lighter
2 = Slightly lighter
3 = Moderately lighter
1 = No variation
2 = Slight variation
3 = Small variation
4 = Moderate variation
5 = Extreme variation
F.
These are descriptive panelist scales for following differences in the cured meat pigment.
1 = Very intense cured color
2 = Intense cured color
3 = Moderate cured color
4 = Medium cured color
5 = Modest cured color
6 = Slight cured color
7 = No cured color
Note: Panelist can record scores to nearest 0.5 point.
1 = Very dark red cured color
2 = Moderately dark red cured color
3 = Slightly dark red cured color
4 = Reddish-pink cured color
5 = Pinkish-red cured color
6 = Slight pinkish-red cured color
7 = Pinkish cured color
8 = Light pinkish cured color
Note: Panelist can record scores to nearest 0.5 point.
1 = No fading
2 = Slight fading
3 = Small fading
4 = Moderate fading
5 = Extreme fading
G.
A line anchored with descriptive terms
| Muscle Darkening in Enhanced Steaks | Fat Color |
|---|---|
| 1 = No darkening | 1 = White |
| 2 = | 2 = Creamy white |
| 3 = Slightly dark | 3 = Slightly yellow |
| 4 = | 4 = Moderately yellow |
| 5 = Moderately dark | 5 = Yellow |
| 6 = | Note: Panelists can record scores to nearest 0.5 point. |
| 7 = Very dark |
| Surface Color Uniformity | Fat Discoloration |
|---|---|
| 1 = Uniform, no two-toning | 1 = No discoloration |
| 2 = Slight two-toning | 2 = Slightly discolored |
| 3 = Small amount two-toning | 3 = Moderately discolored |
| 4 = Moderate two-toning | 4 = Extremely discolored |
| 5 = Extreme two-toning | Note: Panelists can record scores to nearest 0.5 point. |
| Note: Panelists can record scores to nearest 0.5 point. |
| Heat Ring | Purge Characterization |
|---|---|
| 1 = None | 1 = Other (list on scoring sheet) |
| 2 = Slight | 2 = Milky white |
| 3 = Small | 3 = Opaque |
| 4 = Moderate | 4 = Clear |
| 5 = Severe | 5 = Light red |
| Note: Panelists can record scores to nearest 0.5 point. | 6 = Dark red or purple |
| Bone Marrow Color | Off-odor, Immediate and 30 Min After Opening Package |
|---|---|
| 1 = Bright reddish-pink to red | 1 = No off-odor |
| 2 = Dull pinkish-red | 2 = Slight off-odor |
| 3 = Slightly grayish-pink or grayish-red | 3 = Small off-odor |
| 4 = Grayish-pink or grayish-red | 4 = Moderate off-odor |
| 5 = Moderately gray | 5 = Extreme off-odor |
| 6 = All gray or grayish-black | Note: Panelists can record scores to nearest 0.5 point. |
| 7 = Black discoloration |
Iridescence Intensity and Extent
| Intensity | Extent, % |
|---|---|
| 1 = No iridescence | 0% |
| 2 = Very slight iridescence | 1% to 20% |
| 3 = Slight iridescence | 21% to 40% |
| 4 = Moderate iridescence | 41% to 60% |
| 5 = Strong iridescence | 61%–80% |
| 6 = Very strong iridescence | 81% to 100% |
Appendix B: Pictorial Color Guides
A.
Beef Carcass Ribeye Color Guide:
Rpt. AS-515, Iowa Cooperative Extension Service, Meat Laboratory, Iowa State University, Ames, IA 50011, USA (Original Rpt. 336 from New Mexico State University).
AUS-MEAT Beef Lean and Fat Chiller Assessment Colour Standards www.ausmeat.com.au
Japanese Beef Lean and Fat Color Standards:
The Japan Ham & Sausage Cooperative Association, 1-5-6 Ebisu, Shibuya-ku, Tokyo 150-001, Japan. E-mail: kano@hamukumi.or.jp.
Beef Steak Color Guide—Degrees of Doneness
Published by the American Meat Science Association in cooperation with the National Livestock and Meat Board and the U.S. Department of Agriculture Agricultural Research Service (1995). Available from the American Meat Science Association.
Ground Beef Patty Cooked Color Guide:
Dept. of Animal Sciences and Industry, Kansas State University, Manhattan, KS. Available from E. Boyle, Dept. of Animal Sciences and Industry, Weber Hall, Kansas State University, Manhattan, KS 66505, USA.
B.
Procedures to Evaluate Market Hogs:
National Pork Producers Council
Japanese Pork Color Standards:
The Japan Ham & Sausage Cooperative Association, 1-5-6 Ebisu, Shibuya-ku, Tokyo 150- 0013, Japan. E-mail: kano@hamukumi.or.jp.
Pork Chop and Patty Cooked Color Guides:
K-State Research and Extension in cooperation with the National Pork Producers Council and National Pork Board, P.O. Box 10383, Des Moines, IA 50306, USA. Available from E. Boyle, Dept. of Animal Sciences and Industry, Weber Hall, Kansas State University, Manhattan, KS 66505, USA.
C.
Lamb Color Score Guide:
National Livestock and Meat Board. Available from Dept. of Animal Sciences and Industry, Weber Hall, Kansas State University, Manhattan, KS 66506, USA. Available from E. Boyle, Dept. of Animal Sciences and Industry, Weber Hall, Kansas State University, Manhattan, KS 66505, USA.
D.
Cured Meat Color Guide:
Kansas Agricultural Experiment Station. Available from E. Boyle, Dept. of Animal Sciences and Industry, Weber Hall Kansas State University, Manhattan, KS 66505, USA.
E.
Thermometer Calibration Guide:
Flores, N. C., and E. A. E. Boyle. 2000. Kansas Agricultural Experimentation Station and Cooperative Extension Service. Available from E. Boyle, Dept. of Animal Sciences and Industry, Weber Hall, Kansas State University, Manhattan, KS 66505, USA.
Meat Lighting Facts:
Raines, C. R., M. C. Hunt, M. Seyfert, and D. H. Kropf. 2008. Kansas State University and Pennsylvania State University. Available from Department of Animal Science, Manhattan, KS, USA.
Digital Micrometer and Meat in a Kropf Cube:
Cube is a 2.54 cm3 Plexiglas cube covered with oxygen-permeable or -impermeable film for measuring dynamic changes of myoglobin forms.
Gas Measurement in Modified Atmosphere Packages:
Using a gas detector (usually O2, CO2, or CO), with a small needle and some “sticky” patches to help close the hole in the film. Sticky patches are available from most gas instrument and scientific companies.
Kennedy Gauge:
Properly placed close to the seal bar for greatest level of vacuum and accurately measuring vacuum level in meat packages. Available from Kennedy Enterprises, Inc., 4910 Rent-Worth Drive Lincoln, NE 68516, USA. Phone: 800-228-0072.
Kodak Color Separations and Gray Card:
Use for control of color and gray color separations. Include as the first and last pictures in every photo shoot. Store the color separations in an envelope to minimize light-induced color changes.
Appendix C: Equations for Quantifying Myoglobin Redox Forms on Fresh Meat Surfaces
Reflectance measurement closely relates to what the eye and brain perceive. With this non-destructive sampling method, repeated surface measurements over time can be performed on the same sample to measure myoglobin form quantitatively. Moreover, the procedure is rapid and easy to perform. However, considerable attention to detail is needed because reflectance measurements are affected by, among other things, muscle structure, surface moisture, fat content, pigment concentrations, pH, and other inherent muscle properties. In addition, there are many product handling variables such as postmortem age, chilling and temperature history, sample preparation, angle of cutting, packaging, microbial status, etc., that also impact the redox forms of myoglobin and the meat color. Quantitative analyses of specific myoglobin forms are outlined in this document.
There are two established reflectance methodologies for quantifying myoglobin redox forms. One involves using surface reflectance to calculate K/S ratios at isobestic wavelengths for each myoglobin redox form (Francis and Clydesdale, 1975). The other method uses selected wavelengths with a correction factor (Krzywicki, 1979) to calculate percentages of deoxymyoglobin (DMb) and metmyoglobin (MMb) and determines oxymyoglobin (OMb) by difference from 100%. Hernández et al. (2015) reported that the K/S and the reflex attenuance methods gave different proportions of the redox forms of myoglobin; however, both methods resulted in highly significant linear correlations with R2 values of 0.87 for %DMb, 0.98 for %OMb, and 0.94 for %MMb. For anyone doing redox form quantification, this paper is highly recommended.
Estimating DMb, OMb, and MMb (and the equivalent hemoglobin forms) is essential for basic studies of meat pigment stability. However, Ledward (1970) warns that reflectance estimates of the pigment chemical forms were accurate only to ± 6% or 7%. If quantitative determination of myoglobin redox forms on and below the meat surface is of interest, see the oximetry method (Mohan et al., 2010).
A.
Reflectance at wavelengths that are isobestic (equal reflectance for 2 or more of the native forms of myoglobin; see Figure 1) are measured on the meat sample surface and converted to K/S values. Converting reflectance to K/S values makes data more linear and helps account for the scattering (S = scattering coefficient) and absorptive (K = absorbance coefficient) properties of meat (Francis and Clydesdale, 1975). The sample K/S values are put into equations requiring reference values for 100% of the 3 primary meat pigment forms. Please note that K/S reference values for each of the myoglobin forms when converted to 100% vary with conditions, packaging, samples, and instruments unique to each experiment. Therefore, researchers need to determine their own 100% reference values with prepared samples from their experiment rather than use previously published values.
B.
To quantitatively determine the amounts of myoglobin redox forms on meat surfaces, you must have spectrophotometric reflectance values for each pigment form at the isobestic wavelengths (Figure 1). Creating reference standards composed of “100%” DMb, OMb, MMb, or carboxymyoglobin (COMb) on the meat surface is not easy and requires special consideration because each redox state can interconvert rapidly. The forms can be induced chemically or by adjusting the partial pressure of oxygen. When scanning 100% standards, all samples should be scanned through the same packaging film with the same instrument as will be used in the color stability study to remove a potential source of reflectance variation.
- 1.
CRITICAL NOTE #1: If sufficient samples are available, it is highly recommended to dedicate an entire sample, such as a chop, steak, or patty, to pigment form measurements. If there are multiple experimental variables (e.g., muscle, packaging type, etc.), researchers should prepare a reference standard for each combination.
- 2.
CRITICAL NOTE #2: The quantitative methods described below require considerable time and hands-on experience with the creation of meat surfaces containing “100%” of the desired myoglobin redox forms. It is highly recommended that investigators allow ample time to practice and prefect these methods using meat similar to what will be obtained in the research project well before the official research project is started. If this is not done, less than desirable results frequently occur.
- 3.
CRITICAL NOTE #3: Regardless of the myoglobin redox form being created and measured, the scans for reflectance are only as good as the precision and accuracy of the redox form being quantitated. Scans for reflectance will usually be through the packaging film used in the color stability study; therefore, the instrument needs to be standardized using tiles that are covered with a smooth, wrinkle-free layer of the same packaging film used for the samples. In some procedures described below, the film or packaging used to create the desired redox form needs to be removed before the reflectance scans are taken. In addition, it is critical to take several scans immediately for DMb and OMb because they can change rapidly once out of the primary film used to create the redox form. Metmyoglobin and COMb are a bit more stable, but these scans need to be done in a timely, consistent manner on every color measurement from the beginning to the end of the experiment.
- 4.
Metmyoglobin: Select one of the methods.
- a.
Chemical induction: Immerse samples in 1.0% potassium ferricyanide for 1 min, drain, blot surface, and package in oxygen-impermeable film to oxidize at 2°C to 4°C in 1% oxygen for 48 h or longer to maximum formation of MMb before scanning. If the meat is “fresh,” the MMb may get reduced on the surface and a very short distance below the surface, and therefore will not yield the most complete MMb reflectance scan. It is best to start with meat that is older postmortem because it has inherently lower reducing activity, which makes it easier to form 100% MMb on the meat surface.
- b.
Regulation of the oxygen partial pressure: Preferably, start with meat in the DMb state. Store meat in an oxygen-impermeable bag with an atmosphere of 1% oxygen (an oxygen level at which oxidation on myoglobin is highly likely) and 99% nitrogen at room temperature (about 20°C) for 6 h; then, measure the oxygen in the bag (goal is to deplete residual oxygen to 1%, which occurs faster at warmer temperatures). If the residual oxygen is <1%, increase the residual oxygen by injecting a known volume of atmospheric air (which should be about 20.6% oxygen) into the oxygen-impermeable bag using a self-sealing septum, syringe, and a thin needle. With the approximate volume of the bag and the known oxygen concentration, use the following formula to adjust oxygen concentration (volume of the bag × concentration of residual oxygen in the bag) to equal the [desired volume being adjusted × concentration of desired oxygen (1%)]. If the oxygen is >1%, the bag may need re-flushing or start over. Use a gas-to-meat ratio of 3 to 1 or more to avoid myoglobin reduction during storage. The less oxygen absorbed by the meat at the beginning, the easier the conversion; residual oxygen should be checked and adjusted periodically. Maintain 1% oxygen for at least 48 h or longer at 4°C to ensure formation of 100% MMb. When pigment is fully converted, re-package in the film used in the study and scan for MMb. Using stored or aged meat with low MMb reductase activity will mean a more rapid conversion to MMb. Fresh meat can take up to a week to turn brown. Carefully monitor the concentration of oxygen and the development of browning during storage.
- c.
To convert pigment in ground product to MMb, put meat in a bag, flatten the meat with a roller (≈5 mm, thin enough for the atmosphere to penetrate more than 50% of the thickness of the meat), evacuate the air within the bag, flush with 100% nitrogen, and determine and adjust residual oxygen to 1% as described above. Store for pigment conversion on one side. Halfway through the conversion, turn the modified atmosphere package over and loosen the meat from the bag, exposing the other side of the meat to the atmosphere. After 48 hours at 4°C, re-package in oxygen-permeable film and scan several surfaces.
- a.
- 5.
Deoxymyoglobin: Select one of the methods.
- a.
Chemical induction: Immerse samples of uniform dimensions in 0.15% dithionite (sodium hydrosulfite) at room temperature (about 20°C) for 1 to 2 min, drain, blot surface, vacuum package, and allow to reduce for 1 to 2 h at 20°C to maximize conversion to DMb. Repackage in oxygen-permeable film to keep film type the same as that used to measure myoglobin forms for other myoglobin forms and scan immediately. Ground product can be supported using a screen in a beaker.
- b.
Regulation of the oxygen partial pressure: Create a fresh-cut surface that should be essentially 100% DMb by cutting a new cut that is at least 1 cm deep from all edges of the cut, then immediately scan 1 or more times before DMb converts to OMb. Deoxymyoglobin is difficult to retain at 100%. Use color scanning procedures described in the “Instrumental Meat Color Measurement” section.
- c.
Alternate method or combined with 2b: Vacuum package samples (use a very high level of vacuum [>26 mm Hg] to minimize residual oxygen) in a highly oxygen-impermeable vacuum bag and store for 24 to 48 h at 4°C. The conversion of OMb to DMb can be slow, especially at temperatures of −1°C to 4°C. Holding the samples at 10°C–15°C for an hour will greatly facilitate a more complete conversion of OMb to DMb. Usually, MMb forms first from OMb due to the low partial pressure of oxygen and, with time, MMb converts to DMb.
- a.
- 6.
Oxymyoglobin: Select one of the methods.
- a.
Regulation of the oxygen partial pressure: Place samples in a high-oxygen atmosphere, such as a bomb calorimeter or in a bag flushed with 70% to 100% oxygen using at least a 3-to-1 gas-to-meat ratio, then store the flushed bag for 24 to 48 h at 0°C to 2°C (lower temperatures facilitate blooming). Remove the product, cover with the packaging film, and scan immediately to obtain the highest amount of OMb possible.
- b.
For ground product, place the meat in as oxygen-impermeable bag and flatten uniformly with a roller to ≈5 mm thick. Loosen the flattened meat from one side of the bag. Then flush the bag with 70% to 100% oxygen to facilitate formation of OMb. Store the gas-flushed bag for 24 to 48 h at 0°C to 2°C. Halfway through the oxygenation time, flip the bag over and loosen the meat from the package to increase exposure of this side to oxygen. Remove the product from the bag and scan immediately.
- c.
The higher the pH of the meat, the more difficult it is to maximize oxygenation and maintain OMb.
- d.
The colder the storage temperature, the more oxygen will bind to myoglobin because there is less enzyme competition for the oxygen.
- a.
- 7.
Carboxymyoglobin.
- a.
Carboxymyoglobin may have some advantages if used in the K/S ratios for the quantitative analyses of DMb, OMb, and MMb because it is a bit more stable than the other redox forms, and COMb has spectral and reflectance characteristics very similar to OMb. Thus, it might be easier to use COMb in place of OMb when estimating the 3 fresh meat myoglobin native redox forms. This substitution needs to be tested and documented. Below are procedures for creating meat with 100% COMb on the meat surfaces.
- b.
EXERCISE CAUTION when using CO.
- c.
Preferably, start with meat in the DMb state because CO will not bind to OMb and MMb. For example, use whole pieces of meat in a high vacuum or meat packaged in an oxygen-free atmosphere and allow the meat to fully reduce to MMb.
- d.
If the product is ground, flatten it as described in subheading 4c.
- e.
Flushing the packages: Add oxygen scavengers to another bag and quickly transfer the meat from 7c into this bag and flush as completely as possible using a preblend of gases consisting of 0.4% to 1.0% CO (1% would be preferred) and the balance of either nitrogen or a mixture of carbon dioxide and nitrogen.
- f.
Before measuring the COMb formed on the surface, the packages with added oxygen scavengers and meat should be stored for 2 to 3 d at 4°C to facilitate final oxygen removal (and OMb) and the complete reduction of MMb to DMb, thus ensuring that essentially 100% of the surface pigment is converted to COMb.
- a.
C.
Once myoglobin is converted to 100% of each pigment form, record the reflectance at 474, 525, 572, and 610 nm. It is ideal to use the same packing film for all the scans, but this is not always possible, depending on how the myoglobin forms are prepared. Then convert reflectance percentages to K/S values using the following equation: K/S = (1 − R)2 ÷ (2R), where R = percentage reflectance, which should be expressed as a decimal. For example, for a reflectance of 30%, use 0.30, and the K/S calculation should be 0.8167. Many reflectance instruments only record reflectance values at 10-nm intervals. Thus, it will be necessary to integrate the reflectance at 474 using 470 and 480 nm, at 525 using 520 and 530 nm, and at 572 using 570 and 580 nm. First calculate the reflectance values at these wavelengths by integrations, and then convert them to K/S values.
These 100% reference K/S values can then be substituted into the appropriate equation (Figure 2) along with sample K/S values to calculate the percentage of DMb, OMb, or MMb on the sample surface. Equations for myoglobin form estimation were summarized in Hunt (1980). Deoxymyoglobin and MMb determinations have appeared frequently in the literature, and the percentage of OMb is usually determined by difference from 100%. However, determining the percentage of OMb directly using 610 nm (Mancini et al., 2003), which is isobestic for both DMb and MMb, is preferred because OMb content is strongly related to consumer preference (Hunt and Kropf, unpublished data). When determining the percentages of DMb, OMb, and MMb, the percentages may not total 100%. If the percentages do not total 100%, see Mancini et al. (2003) for ways to handle these data.
Case-ready products are often enhanced with one or more added ingredients, including water. Creating reference standards for 100% of each myoglobin form as well as equation-dependent calculations rely on the reflectance properties of meat, which can be influenced by moisture, salt, and other ingredients (Lamkey et al., 1986; Swatland and Barbut, 1999). To maximize accuracy in estimating myoglobin redox forms on the surface of enhanced product, reference standards for 100% DMb, OMb, and MMb should be derived specifically from enhanced product (Ramanathan et al., 2010).
Reflectance methodology for estimating COMb on the surface of meat is not currently available. Nevertheless, spectral characteristics of beef steaks exposed to carbon monoxide suggest a reflectance peak at 500 nm and an absorbance Sorbet wavelength at 420 nm for COMb (Wolfe et al., 1978; Ramanathan et al., 2010). Similar results for tuna muscle have been noted (Smulevich et al., 2007). Suman et al. (2006) published absorbance spectra for equine COMb solutions and concluded that the ratio of absorbance at 543 nm ÷ absorbance at 581 nm could be used to differentiate between COMb and OMb. Additionally, a distinct absorbance valley at 503 nm was reported for 100% COMb samples.
D.
An alternative to using K/S ratios for determining myoglobin forms was presented by Krzywicki (1979). Because 100% conversion of the pigments is not necessary with this method, it does have an advantage. Deoxymyoglobin and MMb are determined and OMb is calculated indirectly by subtracting their combined percentages from 100%. The method is based on the concept of reflex attenuance (A), which is the logarithm of the reciprocal of reflectance. The reflectance is measured at the isobestic wavelengths 474, 525, and 572 nm and at 730 nm, which is referred to as the reflectance of pigment-free meat. Some instruments do not measure reflectance at 730 nm, in which case a reading at 700 nm or any wavelength closer to 730 nm can be used.
Convert the reflectance (R) to reflex attenuance (A) using Equation 1 and insert the A-values in Equation 2 to calculate MMb and in Equation 3 to calculate DMb. Oxymyoglobin is calculated using Equation 4:
where R = reflectance at a specific wavelength expressed as a decimal (0.30 rather than 30%).Francis, F. J., and F. M. Clydesdale. 1975. Food colorimetry: Theory and applications. AVI Publ. Co. Inc., Westport, CT.
Hernández, B., C. Sáenz, C. Alberdi, and J. M. Diñeiro. 2015. Comparison between two different methods to obtain the proportions of myoglobin redox forms on fresh meat from reflectance measurements. J. Food Sci. Tech. Mys. 52:8212–9219. https://doi.org/10.1007/s13197-015-1917-x.
Hunt, M. C. 1980. Meat color measurements. Proceedings of the Reciprocal Meat Conference 33:41–46.
Krzywicki, K. 1979. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. https://doi.org/10.1016/0309-1740(79)90019-6.
Lamkey, J. W., R. W. Mandigo, and C. R. Calkins. 1986. Effect of salt and phosphate on the texture and color stability of restructured beef steaks. J. Food Sci. 51:873–875. https://doi.org/10.1111/j.1365-2621.1986.tb11188.x.
Ledward, D. A. 1970. Metmyoglobin formation in beef stored in carbon dioxide enriched and oxygen depleted atmospheres. J. Food Sci. 35:33–37. https://doi.org/10.1111/j.1365-2621.1970.tb12362.x.
Mancini, R. A., M. C. Hunt, and D. H. Kropf. 2003. Reflectance at 610 nanometers estimates oxymyoglobin content on the surface of ground beef. Meat Sci. 64:157–162. https://doi.org/10.1016/s0309-1740(02)00174-2.
Mohan, A., M. C. Hunt, T. J. Barstow, T. A. Houser, and D. M. Hueber. 2010. Near-infrared oximetry of three post-rigor skeletal muscles for following myoglobin redox forms. Food Chem. 123:456–464. https://doi.org/10.1016/j.foodchem.2010.04.068.
Ramanathan, R., R. A. Mancini, B. M. Naveena, and M. K. R. Konda. 2010. Effects of lactate-enhancement on surface reflectance and absorbance properties of beef longissimus steaks. Meat Sci. 84:219–226. https://doi.org/10.1016/j.meatsci.2009.08.027.
Smulevich, G., E. Droghetti, C. Focardi, M. Coletta, C. Ciaccio, and M. Nocentini. 2007. A rapid spectroscopic method to detect the fraudulent treatment of tuna fish with carbon monoxide. Food Chem. 101:1071–1077. https://doi.org/10.1016/j.foodchem.2006.03.006.
Snyder, H. E. 1965. Analysis of pigments at the surface of fresh beef with reflectance spectrophotometry. J. Food Sci. 30:457–463. https://doi.org/10.1111/j.1365-2621.1965.tb01786.x.
Suman, S. P., R. A. Mancini, and C. Faustman. 2006. Lipid-oxidation-induced carboxymyoglobin oxidation. J. Agr. Food Chem. 54:9248–9253. https://doi.org/10.1021/jf061959u.
Swatland, H. J., and S. Barbut. 1999. Sodium chloride levels in comminuted chicken muscle in relation to processing characteristics and Fresnel reflectance detected with a polarimetric probe. Meat Sci. 51:377–381. https://doi.org/10.1016/s0309-1740(98)00138-7.
Wolfe, S. K., D. A. Watts, and W. D. Brown. 1978. Analysis of myoglobin derivatives in meat or fish samples using absorption spectrophotometry. J. Agr. Food Chem. 26:217–219. https://doi.org/10.1021/jf60215a059.
K/S formulas for calculation of percentage oxymyoglobin (OMb), metmyoglobin (MMb), and deoxymyoglobin (DMb) from reflectance isobestic wavelengths 474, 525, 572, and 610 nm. For OMb, 100% DMb could replace 100% MMb; for MMb, 100% OMb could replace 100% DMb; and for DMb, 100% MMb could replace 100% OMb (Snyder, 1965; Mancini et al., 2003).
Appendix D: Details of Analytical Analyses Related to Meat Color
These protocols detail procedures of commonly used methods for studying myoglobin (Mb). In many cases, researchers can select a procedure and use it as given. In other cases, researchers may need to modify these methods to accommodate special circumstances; in that case, a careful review of the research literature would be prudent.
As in all quantitative analytical chemistry, researchers must give exacting attention to the final calculations, especially using appropriate extinction coefficients, determining the correct dilution factor, and verifying that all units in the equations cancel to the units of measurement desired.
- A.
pH of Pre-rigor Meat
- B.
pH of Post-rigor Meat or Cooked Products
- C.
Total Myoglobin [as Deoxymyoglobin (DMb)] of Fresh or Cooked Meat
- D.
Total Myoglobin (Isobestic Point Assay) of Fresh or Cooked Meat
- E.
Nitrosoheme and Total Heme Content of Cured Meats
- F.
Nitrosoheme and Total Heme Content of Small Samples
- G.
Isolating myoglobin for In Vitro Studies
- H.
Isolating Mitochondria From Beef Skeletal Muscle
- I.
Oxygen Consumption of Intact Muscle or Ground Meat (Normal pH, <5.9)
- J.
Oxygen Consumption of Intact Muscle or Ground Meat (Higher pH, >5.9)
- K.
Metmyoglobin Reducing Ability of Intact or Ground Meat (Normal pH, <5.9)
- L.
Metmyoglobin Reducing Ability of Intact or Ground Meat (Higher pH, >5.9)
- M.
Reduction of Metmyoglobin by Skeletal Muscle Extracts
- N.
Detecting Reflectance of Denatured Globin Hemochromes
- O.
Nitrite Analysis of Cured Meat
- P.
Nitrate Analysis of Cured Meat and Ingredients
- Q.
TBARS for Oxidative Rancidity—Rapid, Wet Method
- R.
TBARS for Oxidative Rancidity—Distillation Method
A.
In pre-rigor muscle, pH slowly decreases because lactic acid produced by glycolysis accumulates. To determine the pH at a given time postmortem, iodoacetate is added to inhibit glycolysis (specifically glyceraldehyde 3-phosphate dehydrogenase), preventing any further production of lactate (Bendall, 1973).
- 1.
150 mM KCl = 11.184 g/L
- 2.
5 mM sodium iodoacetate [(NaIAc) C2H2INaO2 ]: Prepare by dissolving 1.04 g NaIAc in a final volume of 1,000 mL 150 mM KCl. Adjust pH of the solution as needed to 7.0 with a few drops of 0.1 N HCl or 0.1 N NaOH.
The solution should be reasonably fresh (< 3 d old at 3°C). Before analysis, the solution should be allowed to warm to room temperature. Check the pH before use. During analysis, the solution should be stirred on a stir plate to ensure that accurate pH values are obtained. Because it is easy for pre-rigor blended samples to clog electrodes, the pH meter should be verified for accuracy with the 2 standard buffers after every 4 to 5 samples and re-calibrated if necessary.
- 1.
Standardize pH meter with 4.0 and 7.0 buffers before use.
- 2.
Weigh 10 g sample muscle tissue into a blender beaker.
- 3.
Add 100 mL 5 mM NaIAc in 150 mM KCl to the beaker.
- 4.
Blend sample 30 s or until well mixed but not emulsified.
- 5.
Measure pH.
Be sure that the electrode is clean and responds reasonably quickly. Obtaining accurate pH measurements of meat requires proper care and cleaning of electrodes and representative sample collection and sample preparation.
Bendall, J. R. 1973. Postmortem changes in muscle. In: G. H. Bourne, editor, Structure and function of muscle. Vol. 2. Acad. Press, New York, NY. p. 243–309.
B.
- 1.
Standardize pH meter with 4.0 and 7.0 buffers prior to use.
- 2.
Weigh 10 g finely chopped sample into a blender beaker.
- 3.
Add 100 mL de-ionized or distilled water.
- 4.
Blend sample 30 s or until well mixed but not emulsified.
- 5.
Measure pH.
Koniecko, E. S. 1979. Handbook for meat chemists. Avery Publ. Group Inc., Wayne, NJ. p. 53, 54, and 62.
C.
Myoglobin in all forms [deoxymyoglobin, (DMb), oxymyoglobin (OMb), and metmyoglobin (MMb)] is extracted using cold 0.04 M phosphate buffer at pH 6.8 (Warriss, 1979); it is then converted entirely to DMb by adding an excess of a reducing agent, sodium hydrosulfite (dithionite; see note later). The DMb concentration is determined by absorbance of the Soret peak at A433. Note that residual blood hemoglobin will also be extracted and contribute to the total meat pigment content.
Pigment extraction for high-pH meat: Myoglobin quantification from post-rigor meat usually involves a buffer of ≈7.2 pH. However, with pre-rigor muscle or with meat with a higher-than-normal pH, the 7.2 buffer results in lower Mb extraction and turbid filtrates. To help circumvent these issues, Hunt and Hedrick (1977) utilized the method of de Duve (1948), who (1) used an acetate extraction buffer (pH 4.5, 0.01 M) to increase extraction efficiency, (2) adjusted pH to ≈6 with NaOH, and (3) applied a rapid heating treatment (53°C–55°C) to achieve clear filtrates.
- 1.
40 mM potassium phosphate buffer, pH 6.8
- 2.
KH2PO4 = 4.87 g
- 3.
K2HPO4 = 2.48 g
- 4.
1,000 mL distilled/deionized water
- 5.
Sodium hydrosulfite (dithionite)
- 1.
Cut sample into small cubes (or use the sample preparation in Protocol D).
- 2.
Submerge cubes in liquid nitrogen until rapid boiling of liquid nitrogen is complete.
- 3.
Pour small amount of liquid nitrogen into Waring blender.
- 4.
Turn blender on for 2 to 4 s to chill the blender. Blender should be dry to avoid freezing the rotor.
- 5.
Pour pulverized sample onto a clean sheet of paper, and then use the paper to pour sample into a Whirl-Pak bag, removing as much air as possible.
- 6.
Store sample in ultra-low freezer (−60°C) until used.
- 1.
Weigh two (2) 10-g samples of pulverized sample into a Waring blender bowl and record the exact weight of each.
- 2.
Add 90 mL cold potassium phosphate buffer (0.04 M, pH 6.8). Dilution factor is 90 mL + 10 g = 100/10 = 10× dilution.
- 3.
Blend the sample for 1 min.
- 4.
Pour a portion of the uniformly blended sample into a 50-mL centrifuge tube and store at 0°C to 4°C for 1 h for pigment extraction. Note that although there is 100 mL blended sample, only a small volume (3 mL) of supernatant is needed after centrifugation (step 6 below).
- 5.
Centrifuge the samples at 15,000 × g for 30 min at 4°C.
- 6.
Collect the supernatant in a small beaker. Clarify 3 mL supernatant through a syringe filter (0.4-micron pore diameter) into a spectrophotometer cuvette (1 cm width).
- 7.
Add sodium hydrosulfite in a stock solution (see note later) to convert all Mb in the sample to the DMb form.
- 8.
Scan the sample with a scanning spectrophotometer from 700 to 400 nm. The DMb absorption peaks should be within 2 nm of 433 and 556 nm.
All Mb in the sample must be in the DMb form. The Soret peak at 433 nm is a good indicator of DMb. Analyze the sample only if the peak following scanning is within 2 nm of 433 nm. If the peak is within 2 nm of 433 nm, read the absorbance of the peak at 433 nm. Calculate total Mb concentration using the equation that follows.
Molar absorptivity (extinction coefficient) of 1 M DMb solution in a 1-cm path-length cell at 433 nm is 114,000/M (Antonini and Brunori, 1971).
The molecular weight of bovine Mb was 16.949 kDa and was 17.3 kDa for poultry Mb (Joseph et al., 2010). Therefore, an average of 17 kDa can be taken as Mb molecular mass.
Mb concentration (mg/g meat) = A433 × (1 M Mb/114,000) × [(1 mol/L)/M] × (17,000 g Mb/mol Mb) × (1,000 mg/g) × dilution factor of 0.10 L/10 g meat.
% Mb denatured by cooking = [1 − (Mb concentration after heating/Mb concentration before heating)] × 100.
In Mb redox studies, sodium dithionite is used to reduce MMb to DMb before conversion to OMb. Generally, a ratio of 1:10 dithionite to Mb is used. Nevertheless, a higher amount may be required at times. Adding dithionite powder to a Mb solution directly may sometimes result in protein denaturation. To minimize this, a 10% stock solution of dithionite can be used to reduce MMb. For every 1 mL Mb solution at 2.5 mg/mL concentration, 5 microliters 10% dithionite can be added and mixed. If necessary, add another 5 microliters until DMb is formed. This will enhance mixing of dithionite in a Mb solution and will not result in appreciable dilution of the Mb. The dithionite stock solution must be stored in a brown bottle at 4°C and must be prepared fresh daily.
For samples that have smaller concentrations of Mb because of low pigment content or in cooked, denatured samples, the extracted pigment could be converted to MMb by adding a small quantity of potassium ferricyanide. Care should be taken to minimize the quantity added because excess amounts of the oxidant can impart a yellow color to the Mb solution and may interfere with the absorbance. MetMb has a very strong Soret band at 409 nm (Bowen, 1949), which makes it possible to detect smaller concentrations of pigment. Soret peaks often have the greatest absorbance in the Mb absorbance spectra. Hence, the selection of a dilution factor is specific for each peak, the amount of extracted pigment in the test sample, and the maximum absorbance limit of the spectrophotometer.
The dilution factor of 0.11 L/10 g meat was used for cooked steaks in low-oxygen modified atmosphere packaging (MAP). For raw samples, which have more Mb present, 1 mL of supernatant after centrifugation was further diluted with 2 mL cold 0.04 M phosphate buffer, pH 6.8, in a cuvette (dilution factor 3:1). For cooked steak samples packaged in high-oxygen MAP, 10 g pulverized sample was diluted with 50 mL phosphate buffer, for a dilution factor of 0.06 L/10 g meat (Hunt et al., 1999).
Antonini, E., and M. Brunori. 1971. Hemoglobin and myoglobin in their reactions with ligands. North-Holland, Amsterdam, the Netherlands.
Bowen, W. J. 1949. The absorption spectra and extinction coefficients of myoglobin. J. Biol. Chem. 179:235–245.
de Duve, C. 1948. A spectrophotometric method for the simultaneous determination of myoglobin and hemoglobin in extracts of human muscles. Acta Chem. Scand. 2:264–289. https://doi.org/10.3891/acta.chem.scand.02-0264.
Hunt, M. C., and H. B. Hedrick. 1977. Chemical, physical and sensory characteristics of bovine muscle from four quality groups. J. Food Sci. 42:716–720. https://doi.org/10.1111/j.1365-2621.1977.tb12586.x.
Hunt, M. C., O. Sørheim, and E. Slinde. 1999. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 64:847–851. https://doi.org/10.1111/j.1365-2621.1999.tb15925.x.
Joseph, P., S. P. Suman, S. Li, C. M. Beach, and J. R. Claus. 2010. Mass spectrometric characterization and thermostability of turkey myoglobin. LWT-Food Sci. Technol. 43:273–278. https://doi.org/10.1016/j.lwt.2009.08.019.
Warriss, P. D. 1979. The extraction of haem pigments from fresh meat. J. Food Technol. 14:75–80. https://doi.org/10.1111/j.1365-2621.1979.tb00849.x.
D.
This procedure (Faustman and Phillips, 2001) is very similar to Protocol C. Myoglobin in all forms (DMb, OMb, and MMb) is extracted into cold 0.04 M phosphate buffer, pH 6.8 (Warriss, 1979). However, instead of converting the pigment to a particular redox form, the total Mb concentration is determined by absorbance at 525 nm, the isobestic point for all 3 forms of myoglobin. Note that residual blood hemoglobin will also be extracted and contribute to the total meat pigment content.
40 mM potassium phosphate buffer, pH 6.8
- •
KH2 PO4 = 4.87 g
- •
K2 HPO4 = 2.48 g
- •
1,000 mL distilled/deionized water
- 1.
Grind meat through a 1/8-in plate or mince into 3-mm cubes or use the liquid nitrogen method in Protocol C.
- 2.
Weigh duplicate 5-g meat samples and place samples in 50-mL polypropylene tubes.
- 3.
Add 25 mL ice cold phosphate buffer (pH 6.8, 0.04 M) per 5-g sample (Warriss, 1979; Trout, 1989). Dilution factor is 25 mL + 5 g = 30 mL/5 = 6.
- 4.
Homogenize sample for 40 to 45 s at low speed, using the small diameter head of a polytron or similar probe-type homogenizer.
- 5.
Hold the sample in ice (0°C to 4°C) for 1 h.
- 6.
Centrifuge sample at 50,000 × g for 30 min at 5°C. Filter supernatant through Whatman #1 filter paper. A lower g-force may be used, but if the supernatant is turbid (A700 > 0.05), clarify the supernatant through a syringe filter, as described in Protocol C; then measure A525 nm.
- 7.
Measure absorbance at 525 nm (the isobestic point for the 3 forms of myoglobin) to calculate total myoglobin concentration.
Mb concentration (mg/g meat) = (A525 −A700) × (1 mM Mb/7.6) × [(1 mmol/L)/mM] × (17 g Mb/mmol Mb) × (0.03 L/5 g meat; the dilution factor) × (1,000 mg/g), simplified to:
Mb concentration (mg/g meat) = (A525 −A700) × (1 Mm Mb/7.6) × 17 × 6, where 7.6 = millimolar extinction coefficient for Mb at 525 nm and 6 = dilution factor. The molecular masses of Mb vary from 16.9 kDa (livestock Mb) to 17.3 kDa (poultry Mb; Joseph et al., 2010). Therefore, an average of 17 kDa can be used as Mb molecular mass. Absorbance at 700 nm is used to compensate for turbidity (if any) and is therefore subtracted from the absorbance at 525 nm.
Percentage Mb denatured by cooking = [1 − (Mb concentration after heating ÷ Mb concentration before heating)] × 100 (Trout, 1989).
Faustman, C., and A. Phillips. 2001. Measurement of discoloration in fresh meat. In: S. J. Schwartz, editor, Current protocols in food analytical chemistry (Unit F3.3). John Wiley and Sons Inc., New York, NY.
Joseph, P., S. P. Suman, S. Li, C. M. Beach, and J. R. Claus. 2010. Mass spectrometric characterization and thermostability of turkey myoglobin. LWT-Food Sci. Technol. 43:273–278. https://doi.org/10.1016/j.lwt.2009.08.019.
Trout, G. R. 1989. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J. Food Sci. 54:536–540. https://doi.org/10.1111/j.1365-2621.1989.tb04644.x.
Warriss, P. D. 1979. The extraction of haem pigments from fresh meat. J. Food Technol. 14:75–80. https://doi.org/10.1111/j.1365-2621.1979.tb00849.x.
E.
Cured meat pigment is extracted in a solution of 80% acetone and 20% water, including the water content of the sample (Hornsey, 1956). Pigment content (ppm NO-hematin) is calculated based on sample absorbance at 540 nm. Curing efficiency is calculated as the percentage conversion of NO-heme to total heme. Well-cured meats typically have >80% of total pigment in the nitrosoheme form (Pearson and Tauber, 1984). Total heme pigments can also be determined using an acidified acetone solution that extracts heme from all heme proteins, in the form of acid hematin, now commonly referred to as hemin. Total heme concentration is calculated based on sample absorbance at 640 nm.
The nitrosoheme pigment extracted in this assay is very susceptible to fading; thus, considerable care needs to be taken to minimize photochemical oxidation by using reduced lighting and vessels covered with foil.
- 1.
Trim off fatty tissue, and mince the lean in reduced light just before weighing. Conduct all subsequent steps in reduced light.
- 2.
Weigh 10 g minced lean into a tall, 100-mL beaker (to minimize evaporation).
- 3.
Thoroughly mix the lean meat mince with 43 mL a solution containing 40 mL acetone and 3 mL water. Considering a typical 10-g meat sample contains 7 mL water, the extraction solution is 80% acetone, giving maximum nitrosoheme pigment extraction without extracting DMB, OMb, or MMb (Hornsey, 1956).
- 4.
Continue intermittent mixing of the sample for 5 min in reduced light.
- 5.
After 5 min, filter the solution through medium-fast filter paper (Whatman #1 or equivalent) into a 250-mL Erlenmeyer flask.
- 6.
Measure absorption (optical density) of the filtrate in a spectrophotometer at a wavelength of 540 nm using a 1-cm cell. Use 80% acetone/20% water solution for a blank.
NO-heme concentration (as ppm acid hematin) = sample A540 × 290.
- 1.
Why express concentration units as acid hematin?
Hornsey (1956) showed that under acid conditions, NO-heme was oxidized to acid hematin. Thus, acid hematin was used as the standard for determining millimolar absorptivity values at the A540 and A640 peaks. Acid hematin is now more commonly referred to as “hemin.”
- 2.
How was the factor “290” determined?
This factor was derived from the equation A540 = abC, where A540 is sample absorbance, a is absorptivity, b is length of light path (1 cm), and C is concentration of absorbing material (in mM). Absorptivity is a constant dependent on the wavelength of radiation and the nature and molecular weight of the absorbing material. Millimolar absorptivity at a given wavelength, denoted by the symbol EmM, is determined experimentally as the absorbance of a 1 mM solution of the substance in a 1-cm cell. The millimolar absorptivity of NO-heme (oxidized to hemin) at the 540 peak in 80% aqueous acetone is 11.3 (Hornsey, 1956). Hemin molecular weight is 652 Da.
The conversion factors needed to express the concentration in ppm hemin (1 ppm = 1 μg/g), and the dilution factors must also be considered. The dilution factor is the total extraction fluid volume (mL) divided by the sample weight (g). The total extraction fluid volume includes the water content of the sample and the amount of aqueous acetone solution. Most fresh and cooked meats are ∼70% water. Thus, for a 10-g sample containing 7 mL water, the total extraction volume = 7 + 43 mL acetone solution, and the dilution factor = 50/10 = 5.0. If the sample water content differs significantly from 70%, the water content of the acetone solution should be adjusted to yield 80% aqueous acetone during sample extraction (Cornforth, 2001).
Thus, NO-heme concentration C (as ppm hemin) = A540/ab × dilution and conversion factors.
C = A540 × (1 mM NO-hemin/11.3) × (1 mol NO-heme/mol hemin) × [(1 mmol/L)/mM] × (652 mg hemin/mmol hemin) × (50 mL/10 g meat) × (1 g/1,000 mg) × (1 L/1,000 mL) × (106 μg/g).
Simplifying, NO-heme concentration (ppm hemin) = A540 × (1/11.3) × 652 × 5 = A540 × 288.5.
Hornsey rounded the conversion factor to 290.
- 1.
Mix 10-g minced meat sample (70% water) with a solution of 40 mL acetone, 2 mL water, and 1 mL concentrated HCl. This results in an 80:20 acetone:water solution for optimal extraction of heme pigments. For samples with less than 70% water content, add sufficient water to bring the extraction mixture to an 80:20 acetone-to-water ratio.
- 2.
Store and periodically stir solution for 1 h at room temperature before filtering. Acidification of the extraction solution results in extraction heme groups as acid hematin from both fresh and cured meat pigments The acidified acetone solution extracts heme groups in the form of acid hematin from uncured and cured meat pigments (DMb, OMb, MMb, NO-Mb) (Hornsey, 1956).
- 3.
Measure the optical density (1-cm cell) of the filtrate at 640 nm to determine the total heme pigments. Use the solution in step 1 (acidified 80% acetone solution) as a blank.
- 4.
In the estimation of total pigments, absorbance readings may be made for peaks at 512 and 640 nm. The ratio should be <1.9 if oxidation of NO-heme to acid hematin in acidified 80% acetone is complete. Calculate absorbance at 521/640 nm to verify a ratio of <1.9.
- 5.
To express the concentration of total pigments in ppm, multiply the optical density at 640 nm by 680.
Total heme concentration (ppm acid hematin) = sample A640 × 680.
Cure efficiency (%) = (ppm of nitrosoheme ÷ ppm of total pigment) × 100.
Cure efficiency: The percentage of total pigment converted to nitroso pigment; it also indicates the degree of cured color fading.
- 1.
Why express concentration units as acid hematin?
Hornsey (1956) showed that, under acid conditions, heme groups of all heme proteins (including DMb, OMb, MMb, and NO-Mb) were oxidized to acid hematin. Thus, acid hematin is the standard for determining millimolar absorptivity values at the A640. Acid hematin is now more commonly referred to as “hemin.”
- 2.
How was the factor “680” determined?
This factor was derived from the equation A640 = abC, as described previously. The millimolar absorptivity of hemin at the 640 peak in 80% aqueous acetone is 4.8 (Hornsey, 1956). Hemin molecular weight is 652 Da.
Total heme concentration is calculated as shown previously for NO-heme pigment.
Total heme concentration C (as ppm hemin) = A640/ab × dilution and conversion factors.
C = A640 × (1 mM NO-hemin/4.8) × [(1 mmol/L)/mM] × (652 mg hemin/mmol hemin) × (50 mL/10 g meat) × (1 g/1,000 mg) × (1 L/1,000 mL) × (106 μg/g).
Simplifying, NO-heme concentration (ppm hemin) = A640 × (1/4.8) × 652 × 5 = A640 × 679.2.
Hornsey rounded the conversion factor to 680.
Cornforth, D. P. 2001. Spectrophotometric and reflectance measurements of pigments of cooked and cured meats. In: S. J. Schwartz, editor, Current protocols in food analytical chemistry. John Wiley & Sons, Inc., New York, NY. Unit F3.2.
Hornsey, H. C. 1956. Color of cooked cured pork. I. Estimation of the nitric oxide-haem pigments. J. Sci. Food Agr. 23:534–540.
Pearson, A. M., and F. W. Tauber. 1984. Analytical methods. In: A. M. Pearson and F. W. Tauber, editors, Processed meats. 2nd ed. AVI Publishing, Westport, CT. p. 360–361.
F.
Pearson and Tauber (1985) modified Hornsey’s (1956) procedure for analyzing small (2-g) samples, reducing the amount of reagents needed and increasing the number of samples analyzed per day. Samples are placed in capped tubes to prevent evaporation.
- 1.
Acetone-a (aqueous acetone): Place 90 mL distilled water in a 1-L volumetric flask; add spectrophotometric grade acetone, mix and bring to volume.
- 2.
Acetone-b (acidic acetone): Slowly add 20 mL concentrated HCl to 80 mL water.
Transfer the dilute HCl solution to a 1-L volumetric flask, mix, and bring to volume with additional spectrophotometric grade acetone.
- 1.
Do all procedures in subdued light to reduce fading of pigment.
- 2.
Weigh out 2.0-g minced lean meat sample in a 50-mL polypropylene centrifuge tube.
- 3.
Pipet 9.0-mL acetone-a into 50-mL tube, to obtain acetone concentration of 80%.
- 4.
Mix thoroughly with a probe-type homogenizer or a glass rod.
- 5.
Cap the tube to minimize evaporation of acetone, and mix by gentle swirling.
- 6.
Let stand 10 min in the dark, then filter through medium-fast filter paper into a glass test tube.
- 7.
Transfer filtrate into a 1-cm quartz cuvette and read absorbance at 540 nm. (Avoid use of disposable plastic cuvettes. They become opaque upon exposure to acetone). Calculate nitroso pigment concentration as previously described.
- 8.
Prepare another 2.0-g sample, using acetone-b.
- 9.
Macerate and hold 1 h in the dark before filtering.
- 10.
Filter the extract as before and read absorbance at 640 nm. Calculate total pigment and cure efficiency as previously described.
Hornsey, H. C. 1956. Color of cooked cured pork. I. Estimation of the nitric oxide-haem pigments. J. Sci. Food Agr. 23:534–540.
Pearson, A. M., and F. W. Tauber. 1984. Analytical methods. In: A. M. Pearson and F. W. Tauber, editors, Processed meats. 2nd ed. AVI Publishing, Westport, CT. p. 360–361.
G.
Pure myoglobin is sometimes needed, for example, to compare autoxidation rate of Mb among different species. Myoglobin (molecular weight ∼16,949) can be readily purified from skeletal or cardiac muscle. Its red color permits easy visualization during chromatography. This method provides substantial yields of myoglobin, using relatively inexpensive equipment (Faustman and Phillips, 2001; as adapted from earlier procedures of Wittenberg and Wittenberg, 1981, and Trout and Gutzke, 1996).
- 1.
Diced beef muscle trimmed of visible fat and connective tissue
- 2.
Homogenization buffer (10 mM Tris-Cl/1 mM ethylenediaminetetraacetic acid (EDTA), pH 8.0) at 4°C
- 3.
Sodium hydroxide
- 4.
Ammonium sulfate
- 5.
Dialysis buffer (10 mM Tris-Cl/1 mM EDTA, pH 8.0) at 4°C
- 6.
Chromatography elution buffer (5 mM Tris-Cl/1 mM EDTA, pH 8.5) at 4°C
To minimize formation of MMb, homogenization and all subsequent steps should be performed at 0°C to 5°C and high pH (8.0 to 8.5).
Blender, cheese cloth, centrifuge capable of 20,000 × g at 4°C, dialysis tubing (molecular weight cutoff 12,000 to 14,000), Sephacryl S-200 HR chromatography column (30 × 2.5 cm), peristaltic pump. Additional reagents and equipment are needed to assay protein concentration or to calculate myoglobin concentration based on its extinction coefficient.
- 1.
Homogenize 150 g diced muscle in a blender with 450 mL homogenization buffer for 1 to 2 min at high speed.
- 2.
Divide homogenate equally between tubes and centrifuge for 10 min at 3000 × g at 4°C.
- 3.
Pool supernatants, discard precipitate, and adjust pH resulting supernatant to 8.0 using sodium hydroxide.
- 4.
Filter supernatant through 2 layers of cheese cloth to remove lipid and connective tissue particles.
- 1.
Bring filtrate to 70% ammonium sulfate saturation (472 g ammonium sulfate/L filtrate), adjust pH to 8.0 using sodium hydroxide, and stir for 1 h.
- 2.
Divide homogenate equally between tubes and centrifuge 20 min at 18,000 × g at 4°C to remove precipitated proteins.
- 3.
Pool the supernatants and discard precipitate.
- 4.
Bring supernatant from 70% to 100% ammonium sulfate saturation (by adding an additional 228 g ammonium sulfate/L supernatant), adjust pH to 8.0 using sodium hydroxide, and stir for 1 h.
- 5.
Divide homogenate equally between tubes and centrifuge the solution for 1 h at 20,000 × g at 4°C. Discard the supernatant and add 1 or 2 mL ice-cold buffer to aid in recovery of the precipitate.
- 1.
Transfer precipitated myoglobin to dialysis tubing and dialyze against dialysis buffer (1 vol protein, 10 vol buffer) for 24 ho at 4°C, changing buffer every 8 h.
- 2.
Equilibrate a Sephacryl S-200 HR chromatography column with chromatography elution buffer (3 column volumes) using a peristaltic pump.
- 3.
Apply dialysate to column and resolve myoglobin extract with chromatography elution buffer at a flow rate of 60 mL/h. This step separates hemoglobin from myoglobin. Hemoglobin will elute first as a pale red/brown band. Myoglobin will follow as a readily visible dark red band.
- 4.
Collect myoglobin-containing fractions using a fraction collector.
- 1.
Pool all myoglobin-containing fractions. Native polyacrylamide gel electrophoresis can be used to assess the purity of the myoglobin extracts, which should produce a single protein band with a molecular weight of 17 kDa.
- 2.
Concentrate myoglobin solution using centrifugal concentrators.
- 3.
Alternatively (to step 2), bring myoglobin solution to 100% ammonium sulfate saturation (761 g ammonium sulfate/L solution), adjust pH to 8.0, and stir solution for 1 h. Divide solution equally among tubes and centrifuge for 1 h at 20,000 × g at 4°C. Discard supernatants and dialyze myoglobin as described earlier.
- 4.
Alternatively (to steps 2 and 3), the myoglobin may be concentrated by ultrafiltration as described by Trout and Gutzke (1996).
- 5.
Measure protein concentration of myoglobin solution and freeze in aliquots at −80°C.
This myoglobin isolation procedure has relatively low yields and requires 2 to 3 mo of collection to get much volume.
Faustman, C., and A. Phillips. 2001. Measurement of discoloration in fresh meat. In: S. J. Schwartz, editor, Current protocols in food analytical chemistry (Unit F3.3). John Wiley and Sons Inc., New York, NY.
Trout, G. R., and D. A. Gutzke. 1996. A simple, rapid preparative method for isolating and purifying oxymyoglobin. Meat Sci. 43:1–13.
Wittenberg, J. B., and B. A. Wittenberg. 1981. Preparation of myoglobins. Method. Enzymol. 76:29–42.
H.
Mitochondrial isolation consists of 3 steps, cell rupture, homogenization, and centrifugation. The use of proteases with skeletal muscle greatly facilitates the release of the mitochondria and improves yield (Bhattacharya et al., 1991; Frezza et al., 2007).
- 1.
1 M sucrose: Dissolve 342.3 g sucrose in 1 L distilled water; mix well and prepare 20-mL aliquots; store them at −20°C.
- 2.
0.1 M Tris(hydroxymethy)aminomethane (Tris)/3-(N-morpholino)propanesulfonic acid MOPS: Dissolve 12.1 g Tris in 500 mL distilled water, adjust pH to 7.4 using MOPS powder, bring the solution to 1 L and store at 4°C.
- 3.
1 M Tris/HCl: Dissolve 121.14 g Tris in 500 mL distilled water, adjust pH to 7.4 using HCl, bring the solution to 1 L and store at room temperature.
- 4.
1 M EDTA: Dissolve 372.2 g EDTA in 500 mL distilled water and store at 4°C.
- 5.
10% Bovine serum albumin (BSA): Dissolve 10 g BSA in 100 mL distilled water and store at −20°C.
- 6.
1 M Pi: Dissolve 136.1 g KH2PO4 in 500 mL distilled water, adjust pH to 7.4 using Tris powder, bring the solution to 1 L and store at 4°C.
- 7.
10 mM EDTA: Dissolve 2.92 g EDTA in 1 L distilled water and store at 4°C.
- 8.
0.5% BSA: Dissolve 5 g BSA in 1 L distilled water and store at −20°C.
- 9.
Nagarse protease: Prepare a 20 mg % Nagarse (20 mg per 100 mL of the isolation medium).
CRITICAL NOTE: Choice of other proteases (trypsin) depends on investigator preference and protocol as well as the type of muscle used for the isolating mitochondria.
- 10.
Preparation of phosphate buffered saline (PBS) stock solution (Ca2+, Mg2+ free), pH 7.4 (4°C): Prepare 10× PBS using deionized water, and filter through a Millipore filter. Dilute 1:10 just before use with 4°C cold water.
- 11.
For 10×, 1 L PBS, (A) add 500 mL water containing 90 g NaCl; (B) make 500 mL 0.2 M phosphate buffer (20×) by dissolving 13.8 g/500 mL monobasic (add 13.8 g to ∼400 mL deionized H2O and bring volume up to 500 mL in a graduated cylinder), and 14.2 g/500 mL dibasic anhydrous or 26.81 g/500 mL dibasic heptahydrate; (C) to 500 mL dibasic, add enough monobasic (approximately 100 mL or less) to reach pH 7.4; (D) for a 10×,1 L PBS, add 500 mL water containing 90 g NaCl to 500 mL dibasic/monobasic mixture (pH 7.4).
- 12.
Isolation buffer 1 for muscle mitochondria (IBm1): Prepare 1 L IBm1 by mixing 100 mM sucrose, 46 mM KCl, 10 mM EDTA, and 100 mM Tris/HCl. Adjust the pH to 7.4. Bring the volume to 1 L with distilled water.
CRITICAL NOTE: Do not add Nagarse and BSA to this medium.
- 13.
Isolation buffer 2 for muscle mitochondria (IBm2): Prepare 1 L IBm2 by mixing 100 mM sucrose, 46 mM KCl, 10 mM EDTA, 100 mM Tris/HCl, and 0.5% BSA. Adjust the pH to 7.4. Bring the volume to 1 L with distilled water.
CRITICAL NOTE: The Nagarse in medium should be limited to 20 mg in 100 mL solvent.
- 14.
Experimental buffer (for incubating or suspending isolated mitochondria) for muscle mitochondria (EBm): Prepare 1 L EBm by mixing 230 mM mannitol, 70 mM sucrose, 0.02 mM EDTA, 20 mM Tris/HCl, and 5 mM Pi. Adjust the pH to 7.4. Bring the volume to 1 L with distilled water.
CRITICAL NOTE: Use this buffer ONLY for suspension or incubation of isolated mitochondria for further storage, spectrophotometric measurements, and/or enzymatic analytical assays.
- A.
CRITICAL STEP 1: Use of EDTA instead of EGTA chelates also Mg2+, which is extremely abundant in muscle tissue [given the high content in (adenosine triphosphate (ATP)]. Mg2+ can influence mitochondrial function as well as the kinetics of cytochrome c release.
- B.
CRITICAL STEP 2: Wash all glassware 3 times with double distilled water to avoid Ca2+ contamination. Ca2+ overload is the most common cause for the dysfunction of isolated mitochondria.
- C.
CRITICAL STEP 3: Prepare all the buffers the same day of the experiment to avoid bacterial/yeast growth in stored buffers.
- D.
CRITICAL STEP 4: Because pH depends on temperature, measure the pH of all solutions at 25°C.
- A.
- 1.
Remove a 5-g (weigh to 0.1 g) sample of muscle tissue of interest that does not contain any visible fat or connective tissue and cut into small pieces.
- 2.
Using a small beaker, immerse the muscle tissue in 20 mL ice-cold PBS supplemented with 10 mM EDTA.
- 3.
Use scissors to mince the muscle into small pieces.
- 4.
Wash the minced muscle 2 or 3 times with ice-cold PBS supplemented with 10 mM EDTA.
- 5.
Re-suspend the minced muscle in 5 mL ice-cold PBS supplemented with 10 mM EDTA.
- 6.
Centrifuge at 200 × g for 5 min and discard the supernatant.
- 7.
Re-suspend the pellet in 10 mL IBm2.
- 8.
CRITICAL NOTE: The optimal ratio between tissue and isolation buffer ranges from 1:5 to 1:10 (w/v).
- 9.
Homogenize the muscle tissue using a Potter-Elvehjem grinder with Teflon pestle operated at 1,600 rpm; stroke the minced muscle 10 to 20 times.
- 10.
CRITICAL NOTE: Pre-cool the glassware in an ice-bath 5 min before starting the procedure. Homogenization and the following steps must be performed at 4°C to minimize the activation of phospholipases and proteases that might damage the muscle.
- 11.
Transfer the homogenate to a 50-mL polypropylene Falcon tube and centrifuge at 700 × g for 10 min at 4°C.
- 12.
Transfer the supernatant to glass centrifuge tubes and centrifuge at 8,000 × g for 10 min at 4°C.
- 13.
Discard the supernatant and re-suspend the pellet containing mitochondria in 10 mL IBm2. Use a glass rod to loosen the pellet paste.
- 14.
Centrifuge at 8,000 × g for 10 min at 4°C, discard supernatant, and re-suspend the mitochondria (pellet) in 0.3 to 0.5 mL experimental buffer. Use a 200-μL pipette and avoid forming any bubbles during the re-suspension.
CAUTION: Avoid using IBm1 and IBm2 buffers at this stage for suspension.
- 15.
Measure the mitochondrial concentration using one of the Biuret/Bradford/bicinchoninic acid (BCA) assay methods.
Bhattacharya, S. K., J. H. Thakar, P. L. Johnson, and D. R. Shanklin. 1991. Isolation of skeletal muscle mitochondria from hamsters using an ionic medium containing ethylenediaminetetraacetic acid and nagarse. Anal. Biochem. 192:344–349. https://doi.org/10.1016/0003-2697(91)90546-6.
Frezza, C., S. Cipolat, and L. Scorrano. 2007. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2:287–295. https://doi.org/10.1038/nprot.2006.478.
I.
Muscle oxygen consumption (OC) is the ability of the postmortem muscle to consume oxygen, mainly by mitochondria and oxygen-consuming enzymes. OC is an important biochemical property that determines beef color, and it depends on various factors such as pH, muscle type, aging time, and packaging. Hence, researchers should take into account factors that affect OC. There are several ways to quantify OC in meat samples. In general, each method quantifies OMb before and after a specific incubation time, and the disappearance of OMb is the result of converting OMb to either DMb or MMb as neither of these two redox forms will be formed without the consumption of oxygen. In this protocol, a reflectance-based method is utilized.
Freshly cut meat slices are oxygenated (allowed to bloom) for a standardized time and temperature and then vacuum packaged. The decline in OMb is measured as an indicator of the tissue’s ability to consume oxygen. Reflectance spectra over the range 400 to 700 nm are recorded immediately and a second time (often 20 min) in a water bath or incubator kept at 25°C. Oxymyoglobin levels are calculated using the ratio of the reflectance at 610 and 525 nm after K/S transformation. OC is reported as the difference in percentage from the first and last measurements.
Notes:
- a.
The time in the water bath or incubator may need to be increased or decreased for reasons given in procedure 1. It is also possible to simplify the calculations from the detailed 610 methodology to just using K/S ratios (see the alternate calculations that follow).
- b.
Some research reports an actual “rate of oxygen consumption” using percentage changes of OMb per unit of time. This is more laborious and time consuming. With a large number of samples, “oxygen consumption” is often calculated as the “average percentage loss of OMb” relative to the initial level of OMb formed on the sample. The time for deoxygenation of the sample must be standardized. Usually, 20 min is sufficient to detect sample differences.
- c.
The conversion of OMb to DMb is not direct but through MMb. Hence, if researchers are using aged meat samples, they must be aware of the color changes after vacuum packaging.
- 1.
Vacuum packaging machine
- 2.
Polyvinyl chloride (PVC) film
- 3.
Highly oxygen-impermeable vacuum bags (O2 permeability ≤ 0.6 g O/625 cm2/24 h at 0°C)
- 4.
Spectrophotometer that can scan and record surface reflectance from 400 to 700 nm
- 1.
All samples to be assayed must be the SAME temperature, 4°C, for instance. OC will be faster at warmer meat temperatures and bloom development (oxygenation) will be less, whereas samples with lower meat temperatures will bloom more as the competition from oxygen-scavenging enzymes is less.
CRITICAL NOTE: Sample temperature and time have large effects on accuracy and repeatability for OC. As postmortem age of muscle increases, the OC usually decreases; thus, adjustment of the time (step 8) may be necessary. As pH increases, the faster the OC, especially for muscles with more mitochondria. Thus, some preliminary trials are usually necessary to optimize conditions to differentiate fundamental differences for OC due to treatment.
- 2.
Keep all samples at 2°C to 4°C to help ensure uniform oxygenation. For intact, whole muscle, expose a freshly cut meat surface using a sharp knife to remove a 3 cm × 3 cm × 2 cm sample with minimal visible fat or connective tissue. For ground samples, pre-determine the approximate weight of meat needed to be pressd into a square or round containers (e.g., squares or rings cut from plastic piping). It is critical to uniformly press (without over-compacting) the ground meat into comparable sized containers. Again, the visible fat level should be typical of the lean portion of the sample. Avoid dull knives that disrupt surface structure. Also avoid excessive handling and pressing of the blooming surface of ground product (see Madhavi and Carpenter, 1993).
- 3.
If the surface is not a fresh cut, then just before starting the bloom step, remove a thin surface layer to expose fresh tissue.
- 4.
Cover the freshly cut surface with a small piece of oxygen-permeable film to avoid drying. Keep the film (polyvinyl chloride film is commonly used) in one, smooth layer to ensure uniform exposure of the surface to air. Make note of the film’s oxygen permeability.
- 5.
Bloom for 2 h at 2°C to 4°C (or some other standardized time based on preliminary testing). Take care to keep all samples at the same temperature during this step because blooming is very temperature dependent.
- 6.
After bloom, remove the PVC film and place the sample in a pouch with very low oxygen permeability. Quickly vacuum package with high vacuum; keep the vacuum uniform from sample to sample.
- 7.
IMMEDIATELY scan the surface of the sample for reflectance from 400 to 700 nm to determine the initial percentage OMb. The spectrophotometer must be calibrated through the vacuum bag film.
- 8.
To speed up OC, use an incubator or water bath at 25°C. Re-scan the same surface after 20 min (or some standardized time appropriate to the meat being used, such as longer for older meat, maybe less for samples with more mitochondria, more functional mitochondria, and higher-pH meat).
- 9.
Below are 3 ways to calculate OC.
Formula 1.
aThis equation requires the determination of the K/S values for either 100% DMb or 100% MMb. For some meat samples, it may be easier to form 100% of DMb vs. 100% of MMb, or vice versa. Thus, do some preliminary testing to select the redox form that is best for your samples. Be sure to substitute the correct K/S ratios for either 474 nm (DMb) or 572 (MMb).
Equation A:
With Equation A, Formula 1 is used to express OC as the percentage decline of OMb, where a larger percentage means greater tissue OC.
Equation B:
CRITCAL NOTES:
- a)
The literature reports 2 ways to determine percentage OC. Equation A uses 100% myoglobin redox forms for calculating the loss of OMb (Seyfert et al., 2007; English et al., 2016).
- b)
A second faster and more simplified method, Equation B calculates the absolute amount of OMb remaining after the incubation.
- c)
If researchers are using different muscle types, this will help to account for variation in initial OMb formation between muscle types after bloom.
- d)
With Equation B, Formula 1 is used to express OC as the absolute amount of OMb remaining, where a larger percentage means greater tissue OC.
Equation C:
CRITICAL NOTES:
- a)
Changes in OC can also be measured by the decline in OMb pre- and post-incubation. Only OMb is formed and measured using K/S values (Formula 1 is not used).
- b)
The mathematics of Equation C can be confusing because larger ratios indicate less OMb and smaller ratios indicate more OMb, thus the reason for subtracting pre-incubation from post (larger number minus smaller).
- c)
With Equation C, a larger OMb decline in this equation (i.e., the difference of post and pre-incubation) indicates greater OC.
- d)
With this method, values for OMb may occasionally be negative. For negative values, it is recommended that:
- •
A 0 or a very low positive number is entered, or
- •
The equation is reversed by subtracting the post-incubation from the pre-incubation value.
- •
Madhavi and Carpenter (1993) described a reflectance procedure for measuring OC, using a spectrophotometer with reflectance attachment to measure surface OMb levels of vacuum-packaged samples initially and at 5-min intervals (20 min total) at 4°C. Samples were smaller (2.5 × 2.5 × 0.5 cm) to fit in the sample port of the reflectance unit.
Mancini et al. (2003) reported a method using reflectance at 610 nm to directly determine OMb. This is possible because OMb has its unique reflectance at 610 while 610 is isobestic for both DMb and MMb. This method has been used successfully (see King et al., 2011).
English, A. R., G. G. Mafi, D. L. VanOverbeke, and R. Ramanathan. 2016. Effects of extended aging and modified atmospheric packaging on beef top loin steak color. J. Anim. Sci. 94:1727–1737. https://doi.org/10.2527/jas.2015-0149.
King, D. A., S. D. Shackelford, A. B. Rodriguez, and T. L. Wheeler. 2011. Effect of time of measurement on the relationship between metmyoglobin reducing activity and oxygen consumption to instrumental measures of beef longissimus color stability. Meat Sci. 87:26–32. https://doi.org/10.1016/j.meatsci.2010.08.013.
Madhavi, D. L., and C. E. Carpenter. 1993. Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J. Food Sci. 58:939–942. https://doi.org/10.1111/j.1365-2621.1993.tb06083.x.
Mancini, R. A., M. C. Hunt, and D. H. Kropf. 2003. Reflectance at 610 nanometers estimates oxymyoglobin content on the surface of ground beef. Meat Sci. 64:157–162. https://doi.org/10.1016/s0309-1740(02)00174-2.
Seyfert, M., R. A. Mancini, M. C. Hunt, J. Tang, and C. Faustman. 2007. Influence of carbon monoxide in package atmospheres containing oxygen on colour, reducing activity, and oxygen consumption of five bovine muscles. Meat Sci. 75:432–442. https://doi.org/10.1016/j.meatsci.2006.08.007.
J.
A greater postmortem muscle pH can affect muscle structure and biochemical properties. More specifically, a greater pH can lead to less oxygen diffusion and greater mitochondrial activity. High-pH beef may not bloom like normal-pH beef. Hence, OC quantification as changes in OMb level pre- and post-incubation may not represent accurate OC. Furthermore, creating 100% OMb standards are also challenging. Hence, initial OMb formation modified method can be used to quantify OC in high-pH meat.
- 1.
Vacuum packaging machine
- 2.
PVC film
- 3.
Highly oxygen-impermeable vacuum bags (O2 permeability ≤ 0.6 g O/625 cm2/24 h at 0°C)
- 4.
Spectrophotometer that can scan and record surface reflectance from 400 to 700 nm
- 1.
All samples to be assayed must be the same temperature, 4°C, for instance. Otherwise, OC will be faster for samples at warmer temperatures and bloom development (oxygenation) will be less; it will be slower for those at colder temperatures and bloom development will be more.
- 2.
Keep all samples at 2°C to 4°C to help ensure uniform oxygenation. For intact, whole muscle, use a sharp knife to remove a 3 cm × 3 cm × 2 cm sample with minimal visible fat or connective tissue. For ground samples, prepare a comparable sized cube that has been uniformly packed. Again, the visible fat level should be typical of the lean portion of the sample. Avoid dull knives that disrupt surface structure. Also avoid excessive handling and pressing of the blooming surface of ground product (see Madhavi and Carpenter, 1993).
- 3.
If the surface is not a fresh cut, then just before starting the bloom step, remove a thin surface layer to expose fresh tissue.
- 4.
Cover the freshly cut surface with a small piece of oxygen-permeable film to avoid drying. Keep the film (polyvinyl chloride film is commonly used) in one, smooth layer to ensure uniform exposure of the surface to air. Make note of the film’s oxygen permeability.
- 5.
Bloom for 2 h at 2°C to 4°C (or some other standardized time). Take care to keep all samples at the same temperature during this step because blooming is very temperature dependent.
- 6.
After bloom, scan the surface of the sample for reflectance from 400 to 700 nm to determine the initial percentage OMb. The spectrophotometer must be calibrated through the PVC film. Omb is determined as [K/S610 ÷ K/S525].
Percentage OC is reported as resistance to form OMb. A lower K/S610 ÷ K/S525 represents greater OMb formation and thus lower OC.
Madhavi, D. L., and C. E. Carpenter. 1993. Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J. Food Sci. 58:939–942. https://doi.org/10.1111/j.1365-2621.1993.tb06083.x.
K.
Nitrite-induced MMb reducing activity represents total MMb reducing activity of meat (including enzymatic, nonenzymatic, and mitochondria-mediated reduction). Surface pigments are initially oxidized to MMb by soaking of the sample slice in a dilute sodium nitrite solution for 20 min. The slice (1.27 cm thick) is vacuum packaged, and surface percentage MMb is monitored for 2 h at 30°C by measuring reflectance K/S ratios (572/525 nm). Sample reducing ability is defined as the percentage decrease in surface MMb concentration during the incubation period. The decline in MMb is assumed to reflect the tissue’s ability to reduce ferric heme iron. If the pH of the meat is greater than 5.9 as in dark-cutting beef or enhanced beef (such as lactate or ammonium hydroxide), please use the methodology presented in Method L.
- 1.
0.3% (w/w) sodium nitrite solution: Tare a large beaker, weigh 3.0 g NaNO2 into the beaker, and add distilled water to 1,000 g. Make fresh daily. Incubate at room temperature.
- 1.
Remove a 3 cm × 3 cm × 2 cm sample of muscle tissue with no visible fat or connective tissue. For ground meat, use a similar-sized sample that has been uniformly packed together to help avoid crumbling when the sample is immersed.
- 2.
Be sure to orient sample to identify which surface will be evaluated later. This surface may be fresh cut or the surface that was displayed. Submerge sample in 0.3% NaNO2 solution for 20 min at room temperature to induce MMb formation. Ground samples can be placed on a small screen to help lower and raise the cube with minimal crumbling.
- 3.
Remove sample from beaker, and blot to remove excess solution.
- 4.
Retain the three-dimensional shape as much as possible and place the surface for evaluation up in an impermeable bag and vacuum package (a good, uniform vacuum). The vacuum may slightly flatten or round the samples.
- 5.
Scan immediately for reflectance from 400 to 700 nm to determine the initial amount of MMb formed on the surface. Maintain surface integrity.
- 6.
Place sample in an incubator at 30°C and rescan after 2 h to determine the remaining amount of MMb.
%MMb = [K/S572 ÷ K/S525 (for 100% DMb)] −[K/S572 ÷ K/S525 (sample)] ÷ [K/S572 ÷ K/S525 (for 100% DMb)] −[K/S572 ÷ K/S525 (for 100% MMb)] [× 100].
MRA (% of MMb reduced) = [(Initial %MMb −Final %MMb) ÷ Initial %MMb] × 100
Or: use the initial MMb formed as an indicator of MRA (see note below).
Some authors (McKenna et al., 2005; Mancini et al., 2008) indicate that the initial amount of MMb formed by oxidation in sodium nitrite solution is a good indicator of sample MRA. However, King et al. (2011) found that percentage reduction was better than the initial amount of MMb formed. Thus, it is best to collect and statistically analyze both the initial amount of MMb formed and the percentage of MMb reduced over the incubation time.
King, D. A., S. D. Shackelford, A. B. Rodriguez, and T. L. Wheeler. 2011. Effect of time of measurement on the relationship between metmyoglobin reducing activity and oxygen consumption to instrumental measures of beef longissimus color stability. Meat Sci. 87:26–32. https://doi.org/10.1016/j.meatsci.2010.08.013.
Mancini, R. A., M. Seyfert, and M. C. Hunt. 2008. Effects of data expression, sample location, and oxygen partial pressure on initial nitric oxide metmyoglobin formation and metmyoglobin-reducing-activity measurement in beef muscle. Meat Sci. 79:244–251. https://doi.org/10.1016/j.meatsci.2007.09.008.
McKenna, D. R., P. D. Mies, B. E. Baird, K. D. Pfeiffer, J. W. Ellebracht, and J. W. Savell. 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682. https://doi.org/10.1016/j.meatsci.2005.02.016.
L.
A greater pH can enhance mitochondrial and enzyme activity. Hence, less initial metmyoglobin will be formed in high-pH meat. The methodology used in normal pH may not provide realistic MRA (difference between pre- and post-incubation will be lower). Some authors (McKenna et al., 2005; Mancini et al., 2008) indicate that the initial amount of MMb formed by oxidation in sodium nitrite solution is a good indicator of sample MRA. Nitrite-induced initial MMb formation was used as an indicator for MRA in dark-cutting beef (English et al., 2016; McKeith et al., 2016). Further, creating 100% standard for MMb in dark-cutting can be difficult. Hence, reporting initial MMb as K/S572 ÷ K/S525 is recommended.
- 1.
0.3% (w/w) sodium nitrite solution: Tare a large beaker, and weigh 3.0 g NaNO2 into the beaker and add distilled water to 1,000 g. Make fresh daily. Incubate at room temperature.
- 1.
Remove a 3 cm × 3 cm × 2 cm sample of muscle tissue with no visible fat or connective tissue. For ground meat, use a similar sized sample that has been uniformly packed together to help avoid crumbling when the sample is immersed.
- 2.
Be sure to orient sample to identify which surface will be evaluated later. This surface may be fresh cut or the surface that was displayed. Submerge sample in 0.3% NaNO2 solution for 20 min at room temperature to induce MMb formation. Ground samples can be placed on a small screen to help lower and raise the cube with minimal crumbling.
- 3.
Remove sample from beaker, and blot to remove excess solution.
- 4.
Retaining the three-dimensional shape as much as possible, place the surface for evaluation up and cover the surface with a small piece of oxygen-permeable film.
- 5.
Scan immediately for reflectance from 400 to 700 nm to determine the initial amount of MMb formed on the surface.
- 6.
MMb is determined as [K/S572 ÷ K/S525].
MRA reported as resistance to forming MMb. A lower K/S572 ÷ K/S525 represents greater MMb formation and thus a lower MRA.
English, A. R., K. M. Wills, B. N. Harsh, G. G. Mafi, D. L. VanOverbeke, and R. Ramanathan. 2016. Effects of aging on the fundamental color chemistry of dark-cutting beef. J. Anim. Sci. 94:4040–4048. https://doi.org/10.2527/jas.2016-0561.
Mancini, R. A., M. Seyfert, and M. C. Hunt. 2008. Effects of data expression, sample location, and oxygen partial pressure on initial nitric oxide metmyoglobin formation and metmyoglobin-reducing-activity measurement in beef muscle. Meat Sci. 79:244–251. https://doi.org/10.1016/j.meatsci.2007.09.008.
McKeith, R. O., D. A. King, A. L. Grayson, S. D. Shackelford, K. B. Gehring, J. W. Savell, and T. L. Wheeler. 2016. Mitochondrial abundance and efficiency contribute to lean color of dark-cutting beef. Meat Sci. 116:165–173. https://doi.org/10.1016/j.meatsci.2016.01.016.
McKenna, D. R., P. D. Mies, B. E. Baird, K. D. Pfeiffer, J. W. Ellebracht, and J. W. Savell. 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682.
M.
Metmyoglobin reduction by skeletal muscle extracts represents the ability of muscle extract to reduce MMb with the addition of NADH. This represents reducing activity of reductase enzymes such as cytochrome b5 reductase. The researcher should not confuse enzyme activity with total reducing ability as in nitrite-induced MRA. Metmyoglobin reductase activity is monitored as the reduction of MMb to DMb, followed by rapid formation of OMb in an aerobic system. Enzyme activity is calculated based on the increase in absorbance of OMb at 580 nm during the initial linear phase of the reaction (1 to 2 min).
Metmyoglobin reduction can occur by nonenzymatic, enzymatic, and mitochondria-mediated pathways. The methodology for each pathway is different, and the mechanism of action is different. Hence, the researcher should be specific about the MMb reduction method. Care should be taken not to interpret enzymatic MRA as total MRA, i.e., the combination of 3 MRA pathways.
- 1.
Remove a 5-g (weigh to 0.1 g) sample of muscle tissue that does not contain any visible fat or connective tissue; cut sample into small pieces.
- 2.
Homogenize the sample in 20 mL of a 0.2 mM sodium phosphate buffer, pH 5.6 (or the pH of the muscle) for 90 s or until the muscle tissue has been completely disrupted.
- 3.
Centrifuge the homogenate at 35,000 × g in a Beckman ultracentrifuge for 30 min at 4°C.
- 4.
Decant the supernatant into a small beaker and filter 2 to 3 mL with a 0.4-micron syringe filter into a small test tube.
- 5.
Prepare assay solutions:
- a.
5 mM disodium EDTA
- b.
50 mM sodium citrate buffer, pH 5.65 (adjust this pH to the desired pH)
- c.
3.0 mM potassium ferrocyanide
- d.
0.75 mM metmyoglobin (horse skeletal muscle, Sigma M-0630 or purified from pig or bovine (see purification protocol]) in 30 mM sodium phosphate buffer
- e.
1.0 mM NADH (Sigma N-8129)
- a.
- 6.
Turn on the spectrophotometer and warm up for 10 min.
- 7.
If possible, load a spectrophotometric software program that measures the absorbance increase at 580 nm for 180 to 240 s; otherwise, load software manually.
- 8.
Place an empty cuvette in the spectrophotometer cell, and zero the instrument.
- 9.
Add the following reagent amounts to plastic microcuvettes:
- a.
100 μL 5 mM EDTA
- b.
100 μL 50 mM citrate buffer
- c.
100 μL 3.0 mM potassium ferrocyanide
- d.
200 μL 0.75 mM metmyoglobin 200 μL deionized water
- e.
Place each microcuvette in the spectrophotometer cell and simultaneously add 100 μL of 1 mM NADH
- f.
200 μL of filtered muscle extract
- a.
- 10.
Mix well by pipetting and releasing the solution at least 2 times.
- 11.
Note: Add the reagents and mix as quickly as possible because the reaction will begin immediately.
- 12.
Begin measuring the absorbance increase at 580 nm as soon as possible and continue for 180 to 240 s. As metmyoglobin is reduced by the muscle extract, the absorbance at 580 nm will increase.
- 13.
The reducing activity can then be calculated using Beer’s law with the extinction coefficient of 12 × 103 for OMb at 580 nm.
- 14.
Metmyoglobin reductase activity is expressed as nanomoles of metmyoglobin reduced/minute/gram of muscle during the initial linear phase of the time course (usually the first minute or two).
If the absorbance at 580 nm at 0 s is 0 and the absorbance at 60 s is 0.132, then ΔAbs580 nm = 0.132/min. Use Beer’s law to calculate the change in the concentration of metmyoglobin to OMb.
A = Ebc, where
A = Absorbance (or change in absorbance)
b = Path length (1 cm for the plastic microcuvettes)
E = Extinction coefficient (12,000)
C = Concentration in moles/L
0.132 = 12,000 × 1 × c
c = 0.132/12,000
c = 11.0 × 10−6 M/min/5 g of muscle or 11 μM/min/5 g of muscle
Remember this is the change in concentration, not the concentration.
Multiply the change in concentration by the volume in the cuvette to change the concentration to moles.
11 × 10−6 mol/L/min/5 g × 0.0015 L =
16.5 × 10−9 mol reduced/min/5 g of muscle =
16.5 nmol reduced/min/5 g of muscle =
3.3 nmol reduced/min/g of muscle
3.3 nmol of metmyoglobin reduced/min/g = number to report
If you take the change in absorbance over 120 s (2 min) then divide the final number by 2.
Researchers are recommended to avoid the first 20 s of the reaction to allow the reaction to attain a steady state. For example, if the total reaction time is 120 s, better to use time 0 as 21 s and the final time point as 120 s.
Hagler, L., R. I. Coppes Jr., and R. H. Herman. 1979. Metmyoglobin reductase. J. Biol. Chem. 254:6505–6514.
Madhavi, D. L., and C. E. Carpenter. 1993. Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J. Food Sci. 58:939–942. https://doi.org/10.1111/j.1365-2621.1993.tb06083.x.
Mohan, A., M. C. Hunt, T. J. Barstow, T. A. Houser, and D. M. Hueber. 2010. Near-infrared oximetry of three post-rigor muscles for following myoglobin redox forms. Food Chem. 123:456–464. https://doi.org/10.1016/j.foodchem.2010.04.068.
N.
The presence of denatured globin hemochrome pigments is indicated by the presence of reflectance maxima near 528 and 558 nm (Ghorpade and Cornforth, 1993).
- a.
White standard (powdered barium sulfate.)
- b.
Recording spectrophotometer and integrating sphere attachment, with ports for sample and standard.
- c.
Clear polyethylene vacuum bags (1.5-mil thickness).
- 1.
Standardize the recording spectrophotometer to 100% reflectance from 420 to 700 nm, using the white standard (powdered barium sulfate) in both the sample and standard ports of the reflectance attachment.
- 2.
Obtain a uniform meat slice 3 cm × 3 cm (sufficient to completely cover the sample port on the reflectance attachment) and >3 mm thick.
- 3.
To exclude air and minimize fading, rapidly place the fresh slice in a clear polyethylene vacuum bag (1.5-mil thickness). Press the bag against the sample from bottom to top to remove air bubbles. The sample remains in the clear polyethylene bag during reflectance measurement to prevent fading.
- 4.
Place the bagged sample snugly into the sample port of the reflectance sphere, with the freshly sliced surface facing inward (toward the detector). Record surface reflectance (percentage of standard) from 420 to 700 nm. The presence of denatured globin hemochrome pigments is indicated by the presence of reflectance maxima near 528 and 558 nm (Ghorpade and Cornforth, 1993; Cornforth, 2001).
- 5.
Optional: To obtain a difference spectrum between fresh and faded samples, allow a control meat slice to fade in air for 15 to 30 min.
- 6.
Bag the sample and record the reflectance spectra as described for fresh samples.
- 7.
Subtract the baseline spectrum (faded slice) from the fresh sample spectrum.
Cornforth, D. P. 2001. Spectrophotometric and reflectance measurements of pigments of cooked and cured meats. In: S. J. Schwartz, editor, Current protocols in food analytical chemistry [Unit F3.2]. John Wiley and Sons Inc., New York, NY.
Ghorpade, V. M., and D. P. Cornforth. 1993. Spectra of pigments responsible for pink color in pork roasts cooked to 65 or 82°C. J. Food Sci. 58:51–52, 59. https://doi.org/10.1111/j.1365-2621.1993.tb03209.x.
O.
Nitrite ion is extracted into hot water. A portion of the extract is mixed with Greiss reagent [sulfanilamide +N-(1-naphthyl)-ethylenediamine (NED)], forming a pink azo dye with maximum absorbance at 540 nm. The pink color intensity is linearly proportional to the initial nitrite concentration (Beer’s law). Sample nitrite concentration is calculated from the nitrite standard curve, with sample dilution factors considered (AOAC, 1990).
- 1.
NED reagent: Dissolve 0.2 g N-(1-naphthyl)-ethylenediamine-2-HCl in 150 mL of 15% (v/v) acetic acid. Filter if necessary, and store in a brown glass bottle.
- 2.
Sulfanilamide reagent: Dissolve 0.5 g sulfanilamide in 150 mL of 15% (v/v) acetic acid. Filter if necessary, and store in a brown glass bottle.
- 3.
Nitrite standard:
- a.
Stock solution; 1,000 ppm sodium nitrite. Dissolve 1.0 g sodium nitrite in water and dilute to 1 L.
- b.
Intermediate solution; 100 ppm sodium nitrite. Dilute 100 mL stock solution to 1 L with water.
- c.
Working solution; 1 ppm sodium nitrite. Dilute 10 mL intermediate solution to 1 L with water.
- a.
- 4.
Test filter paper for nitrite contamination by analyzing 3 to 4 sheets from box. Filter about 40 mL of water through each sheet. Add 4 mL of sulfanilamide reagent, mix, let stand 5 min, add 4 mL of NED reagent, mix, and wait 15 min. If any sheets test positive, discard the entire box.
- 1.
Weigh 5 g of finely minced tissue and thoroughly mixed sample into 50-mL beaker.
- 2.
Add about 40 mL of 80°C water. Mix thoroughly with glass rod, breaking up all lumps, and transfer to 500-mL volumetric flasks.
- 3.
Wash beaker and rod with successive portions of the hot water, adding all washings to the flask.
- 4.
Add enough hot water to bring volume to about 300 mL, transfer flask to 80°C water bath, and let stand for 2 h, shaking occasionally.
- 5.
Cool to room temperature, dilute to volume with water, and mix again.
- 6.
Filter, add 2.5 mL sulfanilamide reagent to aliquot containing 5 to 50 μg sodium nitrite in 50-mL volumetric flask, and mix.
- 7.
After 5 min, add 2.5 mL of NED reagent, mix, dilute to volume, mix again, and let color develop 15 min.
- 8.
Transfer portion of solution to photometer cell and read A540 against a blank of 45 mL water, 2.5 mL sulfanilamide reagent, and 2.5 mL NED reagent.
- 9.
Prepare standard curve by adding 10, 20, 30, and 40 mL of working sodium nitrite solution to 50-mL volumetric flasks, add 2.5 mL sulfanilamide reagent, mix, and proceed as earlier, beginning with step 7. Standard curve is a straight line to 1 ppm sodium nitrite in final solution.
Sample ppm NaNO2 (μg NaNO2/g sample) = ppm NaNO2 (from the standard curve) × 50/aliquot size (mL) × 500/sample weight (g)
AOAC. 1990. Nitrites in cured meats. Method 973.31. In: K. Helrich, editor, Official Methods of Analysis. 15th ed. AOAC, Arlington, VA. p. 938.
P.
Nitrite and nitrate ions are extracted into hot water. Initial nitrite concentration is determined by the intensity of pink color (A540) upon reaction with Greiss reagents, as described previously. For both nitrate and nitrite determination, sample extracts are incubated with a solution of vanadium(III) and Greiss reagents. The vanadium in acid solution reduces all nitrate ions to nitrite. As nitrite forms, it is captured by Griess reagents, along with pre-existing nitrite. Nitrate concentration = total nitrite (nitrate + nitrite in the vanadium assay) −initial nitrite. The method has been adapted by combining the vanadium and the Greiss reagents into one solution and conducting the assay in spectrophotometer cuvettes.
- 1.
Pour about 200 mL of 0.5 M HCl into a small bottle.
- 2.
Place the bottle on a balance in a hood and directly weigh about 0.5 g vanadium(III) chloride (VCl3) into the bottle (to avoid it sticking to spatulas, weigh dishes, etc.). If undissolved particles remain, filter through a > 2-micron syringe filter.
- 3.
Add about 0.2 g sulfanilamide and 0.01 g N-(1-naphthyl)-ethylenediamine dihydrochloride (NED) and dissolve.
- •
Store the opened bottle of VCl3 over a desiccant such as anhydrous calcium sulfate. VCl3 gives off corrosive fumes when exposed to moist air, but fumes will no longer be released after it is dissolved in the reagent. Vanadium chloride is not classified as toxic or harmful to the environment [material safety data sheet (MSDS) data], unlike cadmium used in older methods.
- •
The VCl3 Greiss reagent solution will keep about a week if refrigerated, but it is sensitive to air and light and is easily oxidized if left at room temperature and uncapped for several days.
- •
Mix the following volumes of sample and reagent directly in semi-microcuvettes, or to scale to suit the cells of the instrument being used.
- •
For 1 to 20 ppm (μg/mL) nitrate nitrogen, mix 20 μL sample with 1,000 μL reagent.
- •
For 1 to 10 ppm nitrate nitrogen, use 45 μL sample and 1,000 μL reagent.
- •
For less than 1 ppm nitrate nitrogen, use 500 μL sample and 500 μL reagent. (Note: 1,000 μL = 1 mL).
- •
If sample concentrations in a new batch are entirely unknown, their concentration range can be quickly estimated by screening several representative samples. Mix equal parts sample and reagent. Do the same for several standards. Heat the samples briefly in an oven or under hot water. Compare the colors to decide on an optimal concentration range.
- •
Pipet the samples into semi-microcuvettes, and then pipet reagent into all cuvettes. Cap the cuvettes with cover caps and invert them gently to mix.
- •
Hold samples at room temperature (20°C to 25°C). Color development slows down after 4 to h and is maximum after 6 to 10 h. Color measurements are taken at A540 against a reagent blank (water). Analyze standards together with the samples to provide a calibration for calculating sample concentrations. Measurements can be taken after 4 to 5 h, but it may be convenient to prepare samples one day and read absorbance the following day. Color may be developed in about 2 h at 60°C, or samples may be mixed in small test tubes and heated at about 100°C for 10 to 15 min to fully develop color.
- •
Meat sample preparation consists of hot water extraction as for nitrite determination. Consider sample dilution factors in calculating nitrate/nitrite concentrations.
- •
This method is modified slightly and described on the NEMI website (NEMI, 2011).
NEMI (National Environmental Methods Index). 2011. National Environmental Methods Index. https://www.nemi.gov.
Q.
In the presence of thiobarbituric acid (TBA), malonaldehyde and other aldehyde products of lipid oxidation (TBA reactive substances [TBARS]) form pink chromogens with maximum absorbance at 532 to 535 nm. However, in the presence of interfering sugars, a yellow chromagen forms, which can be avoided using Tarladgis et al.’s (1960) distillation method.
- d.
TBA stock solution: 0.375% TBA, 15% trichloroacetic acid, and 0.25 N HCl.
- e.
Stock solutions (100 mL) are sufficient for 20 individual tests. Stock solution may be stored at room temperature in the dark (foil-wrapped container).
- 1.
Finely chop or mince a portion of the product of interest. Weigh out duplicate 0.5-g samples.
- 2.
Add 2.5 mL of TBA stock solution to each sample, giving a dilution factor of 6. Mix well.
- 3.
Heat samples 10 min in boiling water in loosely capped tubes (round-bottom Pyrex or polypropylene centrifuge tubes). Caution: Tightly capped tubes may burst during heating. Positive samples turn pink during heating.
- 4.
Cool tubes in tap water.
- 5.
Centrifuge at 5,000 × g for 10 min at 4°C to obtain a clear supernatant.
- 6.
Carefully pipette a portion of the supernatant to a spectrophotometer cuvette. Take care that the solution remains clear.
- 7.
Measure supernatant absorbance at 532 nm against a blank that contains all the reagents but not the meat.
TBARS is expressed as ppm malonaldehyde, using 1.56 × 105/M/cm as the extinction coefficient of the pink TBA chromagen (Sinnhuber and Yu, 1958), as follows:
TBARS number (mg MDA/kg) = sample A 532 × (1 M TBA chromagen/156,000) × [(1 mol/L/M] × (0.003 L/0.5 g meat) × (72.07 g MDA/mol MDA) × 1,000 mg/g) × 1,000 g/kg),
Simplified to: TBARS value (ppm) = sample A 532 × 2.77.
Sinnhuber, R. O., and T. C. Yu. 1958. 2-Thiobarbituric acid method for the measurement of rancidity in fishery products. II. The quantitative determination of malonaldehyde. Food Technol.-Chicago 12:9–12.
Tarladgis, B. G., B. M. Watts, M. T. Younathan, and L. Dugan, Jr. 1960. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 37:44–48. https://doi.org/10.1007/BF02630824.
R.
In this method, the sample is heated in water. Volatile malonaldehyde and other TBA reactive substances (TBARS) are collected by steam distillation. TBA solution is added to an aliquot of the distillate to form the pink TBA chromogen, which is quantified by spectrophotometry (Tarladgis et al., 1960; Koniecko, 1979).
- a.
TBA reagent: Dissolve 1.44 g of 2-TBA (formula wt 144.1) in 450 mL of glacial acetic acid. Bring to volume in 50-mL volumetric flask. Mix and store in the dark (in foil-wrapped container).
- b.
Sulfanilamide reagent: Dissolve 1 g of sulfanilamide in a solution containing 40 mL of concentrated HCl and 160 mL of distilled water.
- c.
Tetra-ethoxy propane (TEP) standard solution in distilled water at a concentration of 2 × 10−8 M of 1,1,3,3-TEP. The solution may be kept refrigerated for 1 wk.
- 1.
Blend 10 g of minced or finely chopped meat sample with 50 mL of distilled water in a Waring blender. Transfer quantitatively to a round-bottomed heating flask (Kjeldahl flask), using 47.5 mL of additional water. Add 2.5 mL of 6 N HCl solution (1, 2 concentrated HCl with water).
- 2.
Add several glass beads to prevent bumping. If foaming is a problem during heating, add an antifoam agent (Dow anti-foam H-10 or equivalent).
- 3.
Heat the flask sufficiently to generate steam. Using a water-cooled distillation apparatus, collect 50 mL of distillate into a graduated cylinder. Time required is about 10 min per sample.
- 4.
Mix distillate well and pipette 5 mL into a 50-mL glass-stoppered flask. Add 5 mL of TBA reagent.
- 5.
Mix and immerse in a boiling water bath for exactly 35 min, along with a blank consisting of 5 mL of distilled water and 5 mL of TBA reagent.
- 6.
Cool flasks for 10 min in tap water. Read absorbance at 538 nm in a spectrophotometer set to zero absorbance for the TBA-water blank.
- 7.
Multiply A538 by a factor of 7.8 to obtain mg malonaldehyde equivalents per 1,000 g meat (ppm MDA). The factor of 7.8 was derived from use of a 10-g sample and 68% recovery of standard from meat (Tarladgis et al., 1960).
- 8.
For validation of the TBA calculations, also determine sample TBA value (ppm MDA) from an MDA standard curve.
- 9.
To prepare the MDA standard curve, pipette 1, 2, 3, 4, and 5 mL of the 2 × 10−8 mol/mL TEP working solution into 50-mL Erlenmeyer flasks. Add sufficient distilled water to bring the total volume to 5 mL. Mix well. No water is needed for the flask containing 5 mL of MDA solution. The TEP concentration is 0.4, 0.8, 1.2, 1.6, and 2.0 × 10−8 mol/5 mL, respectively. TEP is converted to MDA during heating (1:1 basis).
- 10.
Alternatively, to prepare an MDA standard curve with a wider range, add 1, 2, 4, 5, 10, 20, 30, and 40 mL of working stock solution to 50-mL volumetric flasks. Bring to 50-mL volume, mix, and transfer to 50-mL screw-top culture tubes for ease of handling. Transfer a 5-mL portion of each tube to another flask or test tube for the TBA colorimetric reaction, described subsequently. The TEP concentration is 0.2, 0.4, 0.8, 1.0, 2, 4, 6, and 8 × 10−8 mol/5 mL, respectively. This wide-range standard curve is useful for old or rancid samples that may have higher TBA values. See reference by Seyfert et al. (2006).
- 11.
Add 5 mL of TBA reagent to each of the 5-mL standard curve solutions, including a blank (5 mL water). Heat all flasks in a hot water bath at 70°C to 80°C for 35 min. No distillation is needed. Cool flasks in tap water and determine as described previously for meat samples. Plot A538 on the y-axis versus corresponding x-axis values for TEP × 10−8 mol/5 mL. Obtain the linear regression equation for the line of best fit of the standard curve, where the x-axis of the standard curve is TEP concentration (× 10−8 mol/5 mL) and the y-axis is absorbance at 538 nm. Use the sample A538 as the y-value in the regression equation, and solve for x, which is the sample MDA concentration.
Koniecko, E. S. 1979. Handbook for meat chemists. Avery Publ. Group Inc., Wayne, NJ. p. 53, 54, and 62.
Seyfert, M., M. C. Hunt, J. P. Grobbel, S. M. Ryan, D. E. Johnson, and R. A. Monderen. 2006. Potassium lactate and fresh-pork-sausage formulation effects on shelf life in lighted and unlighted display. J. Food Sci. 71:C390–C394. https://doi.org/10.1111/j.1750-3841.2006.00123.x.
Tarladgis, B. G., B. M. Watts, and M. T. Younathan. 1960. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 37:44–48.
Appendix E: Glossary
Absolute BlackA color of the lowest value possessing neither hue nor chroma, closely approximated by looking through a small aperture into a velvet-lined box.
Absolute WhiteA color of the highest value possessing neither hue nor chroma, closely approximated by viewing a piece of freshly cleaned magnesia.
Achromatic ColorsSee “Neutral Colors.”
BloomingA term used to describe the meat exposed to oxygen to form oxymyoglobin, also known as oxygenation of myoglobin. Blooming time is important to specify and is influenced by meat temperature (colder blooms faster).
CarboxymyoglobinThe redox form of myoglobin with carbon monoxide ligated to the 6th position of the heme iron, which is in the ferrous state (Fe2+). Color is cherry red. Carboxymyoglobin is often denoted as COMb.
ChromaThe strength or weakness of a chromatic color, expressed as weak, moderate, or strong; also known as “saturation index.” Calculated as (a*2 + b*2)1/2.
Chromatic ColorsAll colors possessing both hue and chroma.
Commission Internationale de l’Eclairage (CIE) Coordinate SystemA three-dimensional color description system developed by the CIE.
CIE Tristimulus ValuesThe standard color coordinates of the color measuring system developed by the Commission Internationale de l’Eclairage.
ColorA phenomenon of light and visual perception that enables differentiation of otherwise identical objects.
Color AssessmentThe process, following color examination, of deciding whether the difference between the sample and standard—based on either instrumental readings or thoughts—expresses the difference in terms that have a common meaning to all people involved, and then evaluating the expressed difference to decide on the acceptability of the samples.
Color AttributesSee “Color Dimensions.”
Color BalanceAn aesthetic term referring to the feeling of balance, continuity, and fitness found in beautiful color schemes; the physical balance of a color scheme in gray, detected solely by the eye, using disk colorimetry.
Color BlindnessThe inability to distinguish colors properly, associated with an abnormal perception of hue and chroma because of congenital defects or injury to the eye.
Color CoordinatesSee “Color Dimensions.”
Color DescriptionThe delineation of color by using hue, chroma, and value.
Color DimensionsThe attributes of hue, value, and chroma used to describe color.
Color DominanceThe predominance of one hue in a color scheme.
Color ExaminationUse of a source of light to illuminate a sample to be evaluated against a standard and some means of detecting the light coming from the material being examined.
ColorimeterAn instrument in which a sample is viewed in 3 kinds of light, selected so the readings come in the form of 3 numbers, which, with suitable standards, either are directly equal to the 3 CIE tristimulus values or are converted to them by simple calculations.
Color IntensitySee “Chroma.”
Color NotationAn exact description of color using symbols and numerals. For example, a typical maroon is notated as SR 314.
Color Rendering Index (CRI)A quantitative measure of the ability of a light source to reproduce the colors of various objects faithfully compared with an ideal or natural light source. Lighting with a higher CRI should aid visual color panelists to score objects closer to the actual color under a reference illuminant. Technically, CRIs can only be compared for sources that have the same color temperatures. However, as a general rule, the higher the CRIs are the better; light sources with high (85 to 100) CRIs tend to make meat look better than light sources with lower CRIs. However, the CRI is not a particularly good indicator for visual assessments when using lights at <5,000 K. For example, incandescence light (2,700 to 3,000 K has a CRI near 100) makes red meat look good but has too few blue wavelengths, which makes other colors less correct. Conversely, lighting at >5,500 K will have too few red wavelengths to make meat look naturally red.
Color SaturationSee “Chroma.”
Color ScaleA series of colors exhibiting a regular gradation in one dimension while the other 2 dimensions remain constant.
Color Separations GuideA series of different hues that can be used to fine-tune the color of meat photography. When photographing meat in color, one picture should include the meat and a Kodak Color Separations Guide (or any equivalent guide) so that the picture can be adjusted to achieve the most accurate coloration possible. Including a Kodak Gray Scale Guide is also recommended along with the Color Separation Guide. These color guides should be stored in an envelope to reduce light-induced color changes.
Color StandardAn object bearing a specific color against which samples are compared. Such standards may be color photographs or the three-dimensional lower third of the value scale.
Color TemperatureA trait indicating the warmth or coolness of the lighting measured in degrees Kelvin (K) and indicating the light source’s temperature of an ideal black-body radiator that radiates light of comparable hue to that of the light source. Lighting is often characterized by its color temperature. In photographic terms, white balance adjustments to cameras change the color temperature being used by the camera.
Commission Internationale de l’Eclairage (CIE)An international commission devoted to worldwide cooperation and the exchange of information on all matters relating to the science and art of light and lighting, color and vision, photobiology and image technology. See http://www.cie.co.at.
Dark ColorA color of low value that is found in or adjacent to the lower third of the value scale.
Delta Color ChangeAlso known as the total color change or Δ over some specific time period. Generally calculated as Δ* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2. Useful parameter to show total color differences over time. Theoretically, ΔEs of less than 1.0 are not detectable unless the samples are side by side. This parameter is also useful for establishing tolerances for variation in color between samples.
DeoxymyoglobinRedox form of myoglobin that has reduced heme iron (Fe2+). This form has no ligand at the 6th position of the heme iron. Color is purple-red. Essentially no oxygen can be present. In the older literature, this form was called “myoglobin” or “reduced myoglobin,” neither of which is accurate because oxymyoglobin is also reduced myoglobin. Deoxymyoglobin is often denoted as DMb.
Diffuse ReflectanceLight reflected at various angles from the incident light; primarily responsible for the object’s color.
Disk ColorimetryA system for matching specific colors using rapidly spinning disks comprising different colors.
Farnsworth-Munsell 100-Hue TestA subjective test used to screen display study panelists based on the ability to discriminate small differences in hue and to detect color blindness. Details are available at http://www.munsell.eu/html/colour_vision_tests.html. An electronic version of the test is available online at http://www.xrite.com/custom_page.aspx?PageID=77&Lang=en.
Foot-candleEnglish system unit of illumination; 1 foot-candle is 10.76 lux.
GrayA neutral color that possesses neither hue nor chroma.
Gray Scale GuideA series of different shades of gray that can be used to fine-tune meat photography. When photographing meat in color or black and white, one picture should include the meat and a Kodak Gray Scale Guide (or any equivalent guide) so that the picture can be adjusted to achieve the most accurate gray tones possible. Including a Kodak Color Separations Guide is also recommended
along with the Gray Scale Guide. These color guides should be stored in an envelope to reduce light-induced color changes. Hedonic ScalesA type of scale used in sensory analysis with consumer panels where subjects evaluate products using scales of preference, likeness, willingness to purchase, etc. These scales are not appropriate for use with trained visual panels.
HueThe distinctive characteristic of any chromatic color distinguishing it from other hues found between the ends of the spectrum, for example, red, yellow, green, blue, or purple.
Hue AngleThe angle, θ, created by the slope of line b/a in a Hunter color space. H = tan−1 b*/a*. Hue angle values should be in degrees from 0° to 360°. Hue angle has been used for following changes in meat color during storage and display over time that are associated with discoloration.
The mathematics for this calculation have been discussed in the literature for years (see Little, 1975; McLellan et al., 1995). Below are clarifications on the “tricky” calculation.
Critical Warning: Be absolutely sure that your calculations in spreadsheets and statistical programs are giving the correct hue angles. It is highly recommended that sufficient hand calculations of hue angle are done to ensure that electronic calculations are correct.
If a* > 0 and b* > 0; hue angle = arctangent (b*/a*): Value falls between 0 and 90
Example: If a* = 28 and b* = 20, then hue angle = [arctangent (20/28)] = 35.53°
If a* < 0 and b* > 0; hue angle = 180 – arc tan (b*/a*): Value falls between 90 and 180
Example: if a* = – 28 and b* = 20, then the hue angle = [arctangent (–20/28)] = 144.47°
If a* < 0 and b* < 0; hue angle = 180 + arc tan (b*/a*): Value falls between 180 and 270
Example: if a* = –28 and b* = –20, then the hue angle = [arctangent (–28/–20)] = 215.53°
If a* > 0 and b* < 0; hue angle = 360 – arc tan (b*/a*): Value falls between 270 and 360
Example: If a* = 28 and b* = –20, then the hue angle = [arctangent (–20/28)] = 324.47°
Hue CircleA color circle that exhibits a progressively graded series of hues.
HunterLabMajor supplier of equipment and publications for measuring color of food. Hunter Associates Laboratory, Inc., 11491 Sunset Hills Road, Reston, VA 20190-5280, USA. https://www.hunterlab.com
IlluminantA source of light used to illuminate samples or standards. Tristimulus values are calculated from spectral data for specific color temperatures of illuminants, such as Illuminate A, C, or D65.
Instrumental MetamerismA phenomenon that occurs when similar instruments give different readings for exactly the same color because of differences in their spectral response curves.
Isobestic WavelengthA wavelength where the absorbance or reflectance are equal for 2 or more of the myoglobin forms (see Figure 9.1). By selecting the appropriate isobestic wavelengths, the quantitative amount of a myoglobin form can be determined by absorbance or reflectance data, which may need to be converted to/values.
Kennedy GaugeA vacuum gauge set in the center of a steel protective ring (about 9 cm diameter by 2 cm height) to keep the packaging film from being drawn into the vacuum port when a vacuum is drawn. This gauge is very useful for checking or documenting actual vacuum levels being drawn by the vacuum packager and the pressure within sealed packages. Supplied by Kennedy Enterprises, Inc., 4910 Rent-Worth Drive, Lincoln, NE 68518, USA; phone: 800-228-0072.
Konica-MinoltaMajor supplier of equipment and publications for measuring color of food. Konica Minolta Sensing Americas, 101 Williams Drive, Ramsey NJ 97446, USA. https://www.konicaminolta.us
K/S ValuesThe absorption coefficient and scattering coefficient. K/S values are useful for quantifying the proportion of the 3 chemical states of myoglobin present and are calculated from the reflectance (R, expressed as a decimal, not as a percentage) at selected wavelengths using the following equation: K/S = (1 −R)2 ÷ (2R). A reflectance of 40% would have a K/S value of 0.45.
LightThe luminous energy that gives rise to color through stimulation of the retina, which produces nerve currents in the optic nerve and brain.
Light ColorA color of high value found in or adjacent to the upper 3rd of the value scale.
Light PrimariesThree spectrally pure beams of light, which, when blended, allow a large number of colors to be seen.
LuxMetric system unit of illumination equal to 1 lm/m2; 10.76 lux = 1 foot-candle.
LumenUnit of measure for the flow of light through a unit solid angle from a point source of 1 international candle.
Medium ColorA color of medium value located in or adjacent to the middle of the value scale.
Metameric ObjectsObjects that have the same color coordinates and match under a given illuminant but have different spectral reflectance curves and do not match under other illuminants.
MetamerismThe phenomenon of 2 colors matching under a given illuminant but not matching under other illuminants (owing to differences in their spectral reflectance curve) or matching for one observer but not another (owing to differences in their spectral response curves).
MetmyoglobinThe redox form of myoglobin has oxidized (Fe3+) heme iron, which is ligated to water. Color is tan, brown, or tannish-gray and forms readily with very low partial pressures of oxygen. Metmyoglobin is often denoted as MMb.
Metmyoglobin Reducing Ability (MRA)An inherent property of meat where a series of reactions help reduce metmyoglobin. This property seems to be a major factor related to color stability (higher MRA is more stable).
MilA unit of length commonly used for measuring the thickness of packaging films. Also known as a “thou.” One mil equals 0.001 inch or 0.0254 mm; 1 mm = 0.03937 mil or 39.37 thou. Practical measurements often made with a digital caliper with appropriate units and accuracy.
Monochromatic LightLight of only one color.
MyoglobinA water-soluble, sarcoplasmic protein of muscle; the basic pigment in muscle.
Neutral ColorsColors characterized by a complete absence of hue and chroma, such as pure blacks, pure whites, and pure grays.
Nix Sensor Ltd (https://nixsensor.com)Introduced (2015) a small, less expensive colorimeter potential for use in the meat industry for QA, and laboratory color measurement.
ObserverA human or instrument used to detect color differences.
Optical PropertiesProperties involved in the relationship between light and vision, such as visual properties.
Oxygen Consumption (OC)An inherent property of meat where a series of reactions, principally involving the Krebs Cycle enzymes that consume (scavenge) oxygen in meat. OC is responsible for the deoxygenation of oxymyoglobin and the further decrease of oxygen level to 0, allowing the reduction of metmyoglobin to deoxymyoglobin. This term is very similar to oxygen consumption rate (OCR), for which similar measurements are made but a rate of OC per unit time is calculated.
Oxygen Consumption Rate (OCR)See “Oxygen Consumption.”
OxymyoglobinThe redox form of myoglobin that is oxygenated (bloomed) and has oxygen ligated to the 6th position of the heme iron which is Fe2+. Color is bright red. Oxymyoglobin is often denoted as OMb.
Partial PressureIn a mixture of ideal gases, each gas has a partial pressure, which is the pressure that the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture (see table below).
Partial Pressure of Gases in Meat PackagesModified atmosphere packaging often involves a mixture of gases. Each gas’s partial pressure plays a functional role in that package. The table that follows has some useful data about meat packaging where gases are often referred to as a percentage.
PigmentColored matter in an object.
Pigment ConcentrationThe quantity of pigment in muscle, usually in milligrams per gram of wet tissue.
Premature BrowningA condition in which the inner parts of cooked meat turn brown or gray at lower temperatures than expected (55°C to 60°C).
Persistent Pink or RednessA condition in which the interior of cooked, uncured meat retains a red hue at temperatures higher than normally expected for denaturing (loss of red) the raw meat pigments (75°C to 80°C). Frequently occurs with high-pH raw meat. The pinkness sometimes fades in intensity but usually will persist and even intensify in color because of additional oxygenation of the undenatured myoglobin. In some cases, this persistent redness is associated with the formation of a reduced denatured globin hemochrome or NO-hemochrome (cured meat pigment).
Primary ColorsThree colors from which all other colors can be derived, such as red, yellow, and blue.
Principal HuesFive hues that are visually equivalent to each other, such as red, yellow, green, blue, and purple.
Psychometric ScalesVisual scales for measuring color developed through the mental acuity of trained descriptive panels.
Reflection FactorThe percentage of incident light reflected from a sample.
Saturation IndexLength of a radial vector from point of origin to the sample point in a Hunter color space; also known as chroma. For meat, the higher the value, the greater the saturation of red. Saturation index = (a*2 + b*2)1/2; see Little (1975).
ShadeThe color evoked by the mixture of a chromatic pigment with a black pigment or the appearance of that portion of a surface located in a shadow.
Special CharacteristicsCharacteristics of an object related to its light reflectance properties within the visual spectrum.
Spectral Energy Distribution CurveThe curve created by plotting the energy emitted from a given light source against wavelength.
Spectrally Pure ColorThe sensation evoked by spectrally pure light.
Spectral Reflectance CurveThe curve created by plotting the light reflected by an object against wavelength.
Spectral Response CurveThe curve created by plotting the response given by an observer against wavelength.
SpectrophotometerAn instrument used to determine light reflectance or transmission at different wavelengths across the spectrum.
Specular ReflectanceLight reflected at an angle (about 90° from the incident light) that gives a mirror-like appearance, mainly responsible for the gloss of an object.
Standard ObserverThe Standard Observer is related to color matching functions that quantify the sensitivity of red, green, and blue light in the cones of the human eye. The CIE 1931 2° Standard Observer was experimentally developed by color matching when the observer looked through an aperture having 2° field of view. At the time the 1931 2° Standard Observer experiments were conducted, it was thought that cones were concentrated in the foveal region in the eye. Later, it was determined that the cones were spread beyond the fovea. The experiments were re-done in 1964 with a 10° field of view, resulting in the 1964 10° Standard Observer. The 10° Standard Observer is recommended for better correlation with average visual assessments made with large field of view typical of most commercial applications.
TBA2-Thiobarbituric acid, a compound used in testing for lipid oxidation.
TBARSThiobarbituric acid reactive substances, a commonly used name for a lipid oxidation test in which malonaldehyde and other reactive substances are quantified.
ThouA measurement of thickness, 0.001 inch. See “mil.”
TintThe color evoked when a chromatic pigment is mixed with a white pigment or when a small amount of chromatic pigment overlies a white background.
ValueThe lightness or darkness of any color, such as dark, medium, or light.
Value ScaleA series of visually equivalent neutral colors lying between absolute black and absolute white.
Visible SpectrumThe result of a passing beam of light through a glass prism. By this means, the beam of light is broken into an invariable sequence of increasing wavelengths, evident to the eye as a sequence of colors of subtly varying hues of strong chroma.
Visual AssessmentAssessment of color using the visual acuity of sensory panels.
White BalanceA common adjustment for cameras to help record an object’s true appearance by setting the camera’s sensitivity to the color temperature of the object’s environment. White balance settings are usually described as shade, bright sunlight, cloudy, flash, incandescent, fluorescent, etc., and are adjustments of the camera’s color temperature in degrees Kelvin.
Worst-Point Color ScoreA color score derived from the area most severely discolored on a meat surface (single or cumulative area at least 2 cm in diameter).
Typical partial pressures, concentrations, and other gas traits in atmospheric, vacuum, and modified atmosphere packages of meat
| Gas | |||||
|---|---|---|---|---|---|
| Trait | Nitrogen | Oxygen | Argon | Carbon dioxide | Water vapor |
| Gases in air at STP1, % | 78.08 | 20.95 | 0.93 | 0.03 | 1 to 4 |
| Partial pressure of gases in air under STP, mm Hg | 593.4 | 159.2 | 7.1 | 0.2 | — |
| Gases in residual air immediately after vacuum packaging, regardless of level of vacuum Post-vacuum changes2 | 78.1 | 20.9 | 0.9 | 0.03 | — |
STP = standard temperature and pressure. Percentage oxygen decreases about 10% with every km (3,280 ft) above sea level.
Post vacuum packaging; the percentage of gases will go up or down depending on the oxygen consumption of the meat.
Concentration and partial pressure of oxygen in environments with differing percentages of oxygen
| Trait | Oxygen (%, ppm or pressure) values | ||||
|---|---|---|---|---|---|
| Oxygen concentration1 in meat’s atmosphere, % | 0 | 5 | 10 | 15 | Air, 20.9 |
| Oxygen concentration, ppm | 0 | 50,000 | 100,000 | 150,000 | 209,000 |
| Approximate partial pressure of oxygen, mm Hg | 0 | 38.0 | 76.1 | 114.1 | 159.2 |
Metmyoglobin (MMb) forms by oxidation between 1% and 3% oxygen (1,000 and 3,000 ppm).